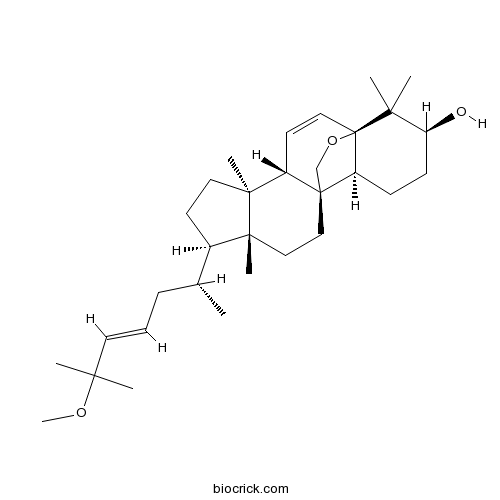
5,19-Epoxy-25-methoxycucurbita-6,23-dien-3-olCAS# 81910-39-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81910-39-6 | SDF | Download SDF |
| PubChem ID | 91884853 | Appearance | Powder |
| Formula | C31H50O3 | M.Wt | 470.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(CC=CC(C)(C)OC)C1CCC2(C1(CCC34C2C=CC5(C3CCC(C5(C)C)O)OC4)C)C | ||
| Standard InChIKey | VQRZZBXVRJGWRS-VHZLPNKGSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5,19-Epoxy-25-methoxycucurbita-6,23-dien-3-ol Dilution Calculator

5,19-Epoxy-25-methoxycucurbita-6,23-dien-3-ol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1245 mL | 10.6225 mL | 21.245 mL | 42.4899 mL | 53.1124 mL |
| 5 mM | 0.4249 mL | 2.1245 mL | 4.249 mL | 8.498 mL | 10.6225 mL |
| 10 mM | 0.2124 mL | 1.0622 mL | 2.1245 mL | 4.249 mL | 5.3112 mL |
| 50 mM | 0.0425 mL | 0.2124 mL | 0.4249 mL | 0.8498 mL | 1.0622 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2124 mL | 0.4249 mL | 0.5311 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderic acid A
Catalog No.:BCN3033
CAS No.:81907-62-2
- Ganoderic acid B
Catalog No.:BCN3034
CAS No.:81907-61-1
- Forskolin J
Catalog No.:BCN4352
CAS No.:81873-08-7
- Seneciocannabine
Catalog No.:BCN2128
CAS No.:81855-31-4
- Neotriptophenolide
Catalog No.:BCN8059
CAS No.:81827-74-9
- Quinocetone
Catalog No.:BCC9133
CAS No.:81810-66-4
- Methimepip dihydrobromide
Catalog No.:BCC7524
CAS No.:817636-54-7
- Praeruptorin B
Catalog No.:BCN4988
CAS No.:81740-07-0
- Bambuterol
Catalog No.:BCC5431
CAS No.:81732-65-2
- PSI-6130
Catalog No.:BCC1870
CAS No.:817204-33-4
- Rhmannioside D
Catalog No.:BCN2362
CAS No.:81720-08-3
- Rehmannioside C
Catalog No.:BCN8183
CAS No.:81720-07-2
- Momordicoside I aglycone
Catalog No.:BCN4353
CAS No.:81910-41-0
- Polyphyllin H
Catalog No.:BCN2834
CAS No.:81917-50-2
- Zofenopril calcium
Catalog No.:BCC5229
CAS No.:81938-43-4
- Z-Ligustilide
Catalog No.:BCC9193
CAS No.:81944-09-4
- 4(15),5,10(14)-Germacratrien-1-ol
Catalog No.:BCN4354
CAS No.:81968-62-9
- Splendoside
Catalog No.:BCN6647
CAS No.:81969-41-7
- Clovin
Catalog No.:BCN7817
CAS No.:81970-00-5
- KW-2478
Catalog No.:BCC2127
CAS No.:819812-04-9
- Hyperxanthone E
Catalog No.:BCN8072
CAS No.:819860-76-9
- Vitedoin A
Catalog No.:BCN6741
CAS No.:819861-40-0
- Lancerin
Catalog No.:BCN2803
CAS No.:81991-99-3
- Khellin
Catalog No.:BCN4356
CAS No.:82-02-0
No significant difference between chiari malformation type 1.5 and type I.[Pubmed:28384597]
Clin Neurol Neurosurg. 2017 Jun;157:34-39.
OBJECTIVE: Chiari malformation Type 1.5 (CM 1.5) was defined as the association of Chiari malformation Type I (CM I) and brainstem herniation. The objective was to demonstrate the difference of clinical features and surgical outcomes between CM 1.5 and CM I. PATIENTS AND METHODS: All CM 1.5 and CM I adult patients who underwent posterior fossa decompression with duraplasty at our institution between 2006 and 2010 were retrospectively reviewed. Clinical characteristics, imaging features, and long-term outcomes were compared between CM 1.5 and CM I patients. RESULTS: A total of 142 adult patients were enrolled, including 27 CM 1.5 and 115 CM I patients. The average follow-up period was 102 months. Age at diagnosis was significantly younger in CM 1.5 group than CM I group (p=0.039). And the degree of tonsillar herniation was significantly more severe in CM 1.5 group than CM I group (p<0.001). There was no significant difference in other clinical and imaging characteristics. Moreover, improvement of symptoms was observed in 21 CM 1.5 patients (77.8%) and 94 CM I patients (81.7%), and no significant difference was detected (p=0.637). There was no significant difference in the resolution of syringomyelia between CM 1.5 (72.7%) and CM I (76.5%) patients, either (p=0. 710). CONCLUSIONS: Although CM 1.5 patients presented with brainstem herniation and more severe tonsillar herniation, other clinical and imaging features and surgical outcomes were similar with CM I patients. We think CM 1.5 is just a subtype of CM I, rather than a unique type of Chiari malformations.
The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5.[Pubmed:28384801]
JAMA Psychiatry. 2017 May 1;74(5):520-526.
Importance: Recognition that adult attention-deficit/hyperactivity disorder (ADHD) is common, seriously impairing, and usually undiagnosed has led to the development of adult ADHD screening scales for use in community, workplace, and primary care settings. However, these scales are all calibrated to DSM-IV criteria, which are narrower than the recently developed DSM-5 criteria. Objectives: To update for DSM-5 criteria and improve the operating characteristics of the widely used World Health Organization Adult ADHD Self-Report Scale (ASRS) for screening. Design, Setting, and Participants: Probability subsamples of participants in 2 general population surveys (2001-2003 household survey [n = 119] and 2004-2005 managed care subscriber survey [n = 218]) who completed the full 29-question self-report ASRS, with both subsamples over-sampling ASRS-screened positives, were blindly administered a semistructured research diagnostic interview for DSM-5 adult ADHD. In 2016, the Risk-Calibrated Supersparse Linear Integer Model, a novel machine-learning algorithm designed to create screening scales with optimal integer weights and limited numbers of screening questions, was applied to the pooled data to create a DSM-5 version of the ASRS screening scale. The accuracy of the new scale was then confirmed in an independent 2011-2012 clinical sample of patients seeking evaluation at the New York University Langone Medical Center Adult ADHD Program (NYU Langone) and 2015-2016 primary care controls (n = 300). Data analysis was conducted from April 4, 2016, to September 22, 2016. Main Outcomes and Measures: The sensitivity, specificity, area under the curve (AUC), and positive predictive value (PPV) of the revised ASRS. Results: Of the total 637 participants, 44 (37.0%) household survey respondents, 51 (23.4%) managed care respondents, and 173 (57.7%) NYU Langone respondents met DSM-5 criteria for adult ADHD in the semistructured diagnostic interview. Of the respondents who met DSM-5 criteria for adult ADHD, 123 were male (45.9%); mean (SD) age was 33.1 (11.4) years. A 6-question screening scale was found to be optimal in distinguishing cases from noncases in the first 2 samples. Operating characteristics were excellent at the diagnostic threshold in the weighted (to the 8.2% DSM-5/Adult ADHD Clinical Diagnostic Scale population prevalence) data (sensitivity, 91.4%; specificity, 96.0%; AUC, 0.94; PPV, 67.3%). Operating characteristics were similar despite a much higher prevalence (57.7%) when the scale was applied to the NYU Langone clinical sample (sensitivity, 91.9%; specificity, 74.0%; AUC, 0.83; PPV, 82.8%). Conclusions and Relevance: The new ADHD screening scale is short, easily scored, detects the vast majority of general population cases at a threshold that also has high specificity and PPV, and could be used as a screening tool in specialty treatment settings.
Reliability and validity of the DSM-5 Anxious Distress Specifier Interview.[Pubmed:28384524]
Compr Psychiatry. 2017 Jul;76:11-17.
BACKGROUND: To acknowledge the clinical significance of anxiety in depressed patients, DSM-5 included an anxious distress specifier for major depressive disorder (MDD). In the present report we describe the reliability and validity of a semi-structured interview assessing the features of the anxious distress specifier. Our goal was to develop an instrument that could be used for both diagnostic and outcome measurement purposes. METHODS: One hundred seventy-three psychiatric patients with MDD were interviewed by a trained diagnostic rater who administered the Structured Clinical Interview for DSM-IV (SCID) supplemented with questions from the DSM-5 Anxious Distress Specifier Interview (DADSI). Inter-rater (n=25) and test-retest (n=25) reliability of the DADSI was examined in separate groups of patients. The patients were rated on clinician rating scales of depression, anxiety and irritability, and patients completed self-report measures of these constructs. Sensitivity to change was examined in 16 patients. RESULTS: Approximately three-quarters of the depressed patients met the criteria for the anxious distress specifier (78.0%, n=135). The DADSI had excellent joint-interview reliability and good test-retest reliability. DADSI total scores were more highly correlated with other clinician-rated and self-report measures of anxiety than with measures of depression and anger. DADSI scores were significantly higher in depressed outpatients with a current anxiety disorder than depressed patients without a comorbid anxiety disorder. The DADSI was sensitive to improvement. CONCLUSION: The DADSI is a reliable and valid measure of the presence of the DSM-5 anxious distress specifier for MDD as well as the severity of the features of the specifier.
PM2.5-induced alterations of cell cycle associated gene expression in lung cancer cells and rat lung tissues.[Pubmed:28384515]
Environ Toxicol Pharmacol. 2017 Jun;52:77-82.
The aim of the current study was to investigate the expression of cell cycle-associated genes induced by fine particulate matter (PM2.5) in lung cancer cell line and tissues. The pulmonary lymph node metastasis cells (H292) were treated with PM2.5in vitro. Wistar rats were used to perform an in vivo study. Rats were randomly assigned to experiment and control groups and those in the experiment group were exposed to PM2.5 once every 15 d, while those in the control group were exposed to normal saline. The cell cycle-associated genes expression was analyzed by real-time PCR. Trachea and lung tissues of rats were processed for scanning electron microscopic (SEM) examinations. Exposure of H292 cells to PM2.5 dramatically increased the expressions of p53 and cyclin-dependent kinase 2 (CDK2) after 24h of exposure (p<0.01) and markedly increased the expressions of the cell division cycle 2 (Cdc2) and cyclin B after 48h of exposure (p<0.01), while those genes expressions were significantly reduced after 72h of exposure, at which time the expression of p21 was predominant (p<0.01). In vivo studies further demonstrated these results. The results of SEM suggested that both of the trachea and lung tissues were damaged and the degree of damage was time-dependent. In conclusion, PM2.5 can induce significantly alterations of p53 and CDK2 in the early phase, Cdc2 and cyclin B in mid-term and p21 in long-term exposure. The degree of PM2.5-induced damage to the trachea and lung tissue was time-dependent.


