XylotrioseCAS# 47592-59-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 47592-59-6 | SDF | Download SDF |
| PubChem ID | 91873341 | Appearance | White crystalline powder |
| Formula | C15H26O13 | M.Wt | 414.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
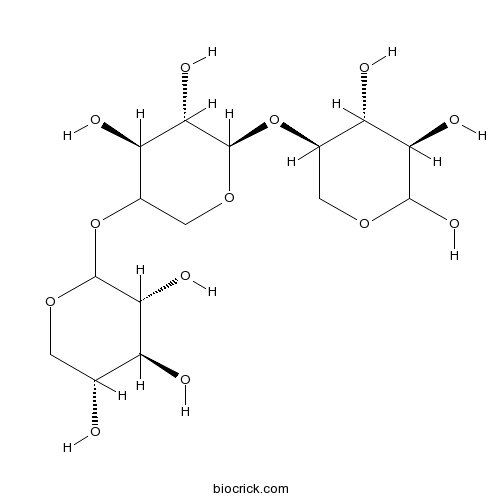
| Chemical Name | (3R,4S,5R)-2-[(4S,5S,6R)-4,5-dihydroxy-6-[(3R,4R,5R)-4,5,6-trihydroxyoxan-3-yl]oxyoxan-3-yl]oxyoxane-3,4,5-triol | ||
| SMILES | C1C(C(C(C(O1)OC2COC(C(C2O)O)OC3COC(C(C3O)O)O)O)O)O | ||
| Standard InChIKey | JCSJTDYCNQHPRJ-QZPPXLSYSA-N | ||
| Standard InChI | InChI=1S/C15H26O13/c16-4-1-25-14(11(21)7(4)17)28-6-3-26-15(12(22)9(6)19)27-5-2-24-13(23)10(20)8(5)18/h4-23H,1-3H2/t4-,5-,6?,7+,8+,9-,10-,11-,12+,13?,14?,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Xylotriose Dilution Calculator

Xylotriose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4134 mL | 12.0668 mL | 24.1336 mL | 48.2672 mL | 60.334 mL |
| 5 mM | 0.4827 mL | 2.4134 mL | 4.8267 mL | 9.6534 mL | 12.0668 mL |
| 10 mM | 0.2413 mL | 1.2067 mL | 2.4134 mL | 4.8267 mL | 6.0334 mL |
| 50 mM | 0.0483 mL | 0.2413 mL | 0.4827 mL | 0.9653 mL | 1.2067 mL |
| 100 mM | 0.0241 mL | 0.1207 mL | 0.2413 mL | 0.4827 mL | 0.6033 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- 2-Methylthioadenosine diphosphate trisodium salt
Catalog No.:BCC5794
CAS No.:475193-31-8
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- Lycorine
Catalog No.:BCN2409
CAS No.:476-28-8
- Chelidonine
Catalog No.:BCN2463
CAS No.:476-32-4
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
- Corydine
Catalog No.:BCN2669
CAS No.:476-69-7
- Boldine
Catalog No.:BCN5534
CAS No.:476-70-0
- VO-Ohpic trihydrate
Catalog No.:BCC2043
CAS No.:476310-60-8
- Eupaglehnin C
Catalog No.:BCN7118
CAS No.:476630-49-6
- Lushanrubescensin H
Catalog No.:BCN3235
CAS No.:476640-22-9
- 6,9,10-Trihydroxy-7-megastigmen-3-one
Catalog No.:BCN1435
CAS No.:476682-97-0
- 3-(2-Benzothiazolylthio)propionic acid
Catalog No.:BCC8586
CAS No.:4767-00-4
- Boc-Tyr(2-Br-Z)-OH
Catalog No.:BCC3460
CAS No.:47689-67-8
- Lycorenine
Catalog No.:BCN2507
CAS No.:477-19-0
A plasmid borne, functionally novel glycoside hydrolase family 30 subfamily 8 endoxylanase from solventogenic Clostridium.[Pubmed:29626157]
Biochem J. 2018 May 4;475(9):1533-1551.
Glycoside hydrolase family 30 subfamily 8 (GH30-8) beta-1,4-endoxylanases are known for their appendage-dependent function requiring recognition of an alpha-1,2-linked glucuronic acid (GlcA) common to glucuronoxylans for hydrolysis. Structural studies have indicated that the GlcA moiety of glucuronoxylans is coordinated through six hydrogen bonds and a salt bridge. These GlcA-dependent endoxylanases do not have significant activity on xylans that do not bear GlcA substitutions such as unsubstituted linear xylooligosaccharides or cereal bran arabinoxylans. In the present study, we present the structural and biochemical characteristics of xylanase 30A from Clostridium acetobutylicum (CaXyn30A) which was originally selected for study due to predicted structural differences within the GlcA coordination loops. Amino acid sequence comparisons indicated that this Gram-positive-derived GH30-8 more closely resembles Gram-negative derived forms of these endoxylanases: a hypothesis borne out in the developed crystallographic structure model of the CaXyn30A catalytic domain (CaXyn30A-CD). CaXyn30A-CD hydrolyzes xylans to linear and substituted oligoxylosides showing the greatest rate with the highly arabinofuranose (Araf)-substituted cereal arabinoxylans. CaXyn30A-CD hydrolyzes xylooligosaccharides larger than Xylotriose and shows an increased relative rate of hydrolysis for xylooligosaccharides containing alpha-1,2-linked arabinofuranose substitutions. Biochemical analysis confirms that CaXyn30A benefits from five xylose-binding subsites which extend from the -3 subsite to the +2 subsite of the binding cleft. These studies indicate that CaXyn30A is a GlcA-independent endoxylanase that may have evolved for the preferential recognition of alpha-1,2-Araf substitutions on xylan chains.
High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerisation.[Pubmed:29433045]
Bioresour Technol. 2018 May;256:110-117.
In the present work, xylan from arecanut husk was extracted using 2 stage alkaline pretreatment process. In first step, biomass was incubated in alkali at different temperatures (25 degrees C, 50 degrees C and 65 degrees C), alkali concentrations (5%, 10%, 15% and 20% w/v), and incubation periods (8h, 16h and 24h) and evaluated for xylan recovery. It was observed that 40-52% of available xylan could be recovered using 10% alkali when incubated for 8-24h at 65 degrees C. Subsequently, the alkali pretreatment operating conditions which provided good xylan recovery were processed further using hydrothermal treatment to extract more xylan. For maximum xylan recovery (>90%), best operating conditions were identified when biomass was treated under hydrothermal treatment (1, 1.5 and 2h) with varying incubation periods (8, 16, 24h) and alkali concentrations (5%, 10%) using full factorial design. Incubating arecanut husk with 10% w/v NaOH, at 65 degrees C for a period of 8h, followed by hydrothermal treatment at 121 degrees C for 1h helped recover >94% xylan. In the next step, enzymatic hydrolysis process was optimized to recover maximum XOS (Optimized condition: 50 degrees C, pH 4 and 10U enzyme dose). The hydrolysate comprised of xylobiose: 25.0+/-1.2g/100g xylan ( approximately 71% of XOS), Xylotriose: 9.2+/-0.65g/100g xylan (26.2% of XOS) and xylotetrose: 0.9+/-0.04g/100g xylan (2% of XOS). The developed process enables to reduce alkali consumption for high recovery of xylan from biomass with relatively higher lignin content for its valorisation into a potential prebiotic oligosaccharide.
Fast automated online xylanase activity assay using HPAEC-PAD.[Pubmed:29184998]
Anal Bioanal Chem. 2018 Jan;410(1):57-69.
In contrast to biochemical reactions, which are often carried out under automatic control and maintained overnight, the automation of chemical analysis is usually neglected. Samples are either analyzed in a rudimentary fashion using in situ techniques, or aliquots are withdrawn and stored to facilitate more precise offline measurements, which can result in sampling and storage errors. Therefore, in this study, we implemented automated reaction control, sampling, and analysis. As an example, the activities of xylanases on xylotetraose and soluble xylan were examined using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The reaction was performed in HPLC vials inside a temperature-controlled Dionex AS-AP autosampler. It was started automatically when the autosampler pipetted substrate and enzyme solution into the reaction vial. Afterwards, samples from the reaction vial were injected repeatedly for 60 min onto a CarboPac PA100 column for analysis. Due to the rapidity of the reaction, the analytical method and the gradient elution of 200 mM sodium hydroxide solution and 100 mM sodium hydroxide with 500 mM sodium acetate were adapted to allow for an overall separation time of 13 min and a detection limit of 0.35-1.83 mg/L (depending on the xylooligomer). This analytical method was applied to measure the soluble short-chain products (xylose, xylobiose, Xylotriose, xylotetraose, xylopentaose, and longer xylooligomers) that arise during enzymatic hydrolysis. Based on that, the activities of three endoxylanases (EX) were determined as 294 U/mg for EX from Aspergillus niger, 1.69 U/mg for EX from Bacillus stearothermophilus, and 0.36 U/mg for EX from Bacillus subtilis. Graphical abstract Xylanase activity assay automation.
Improving Hydrolysis Characteristics of Xylanases by Site-Directed Mutagenesis in Binding-Site Subsites from Streptomyces L10608.[Pubmed:29533991]
Int J Mol Sci. 2018 Mar 13;19(3). pii: ijms19030834.
The preparation of oligosaccharides via xylan hydrolysis is an effective way to add value to hemicellulosic material of agricultural waste. The bacterial strain Streptomyces L10608, isolated from soil, contains genes encoding xylanases of glucoside hydrolase family 10/11 (GH10/11), and these have been cloned to catalyze the production of xylooligosaccharide (XOS). To improve the XOS proportion of hydrolysates produced by xylanase, four amino acid residues were substituted by site-directed mutagenesis, and the mutant genes were overexpressed in Escherichia coli. Mutations replaced the codons encoding Asn214 (+2) and Asn86 (-2) by Ala and removed the Ricin B-lectin domain in GH10-xyn, and mutants Y115A (-2) and Y123A (-2) were produced for GH11-xyn. Interestingly, GH10-N86Q had significantly increased hydrolysis of XOS and almost eliminated xylose (X1) to <2.5%, indicating that the -2 binding site of GH10-xyn of L10608 is required for binding with Xylotriose (X3). The hydrolytic activity of GH10-N86Q was increased approximately 1.25-fold using beechwood xylan as a substrate and had high affinity for the substrate with a low Km of about 1.85 mg.mL(-1). Otherwise, there were no significant differences in enzymatic properties between GH10-N86Q and GH10-xyn. These mutants offer great potential for modification of xylanase with desired XOS hydrolysis.
Thermostability improvement of a Talaromyces leycettanus xylanase by rational protein engineering.[Pubmed:29127292]
Sci Rep. 2017 Nov 10;7(1):15287.
Thermophilic xylanases with high catalytic efficiency are of great interest in the biofuel, food and feed industries. This study identified a GH11 xylanase gene, Tlxyn11B, in Talaromyces leycettanus JCM12802. Recombinant TlXyn11B produced in Pichia pastoris is distinguished by high specific activity (8259 +/- 32 U/mg with beechwood xylan as substrate) and excellent pH stability (from 1.0 to 10.5). The beechwood xylan hydrolysates consisted mainly of xylobiose, Xylotriose and xylotetraose, thus TlXyn11B could be used for the production of prebiotic xylooligosaccharide. By using the structure-based rational approach, the N-terminal sequence of TlXyn11B was modified for thermostability improvement. Mutants S3F and S3F/D35V/I/Q/M had elevated T m values of 60.01 to 67.84 degrees C, with S3F/D35I the greatest. Homology modeling and molecular dynamics (MD) simulation analysis revealed that the substituted F3 and I35 formed a sandwich structure with S45 and T47, which may enhance the overall structure rigidity with lowered RMSD values. This study verifies the efficiency of rational approach in thermostability improvement and provides a xylanase candidate of GH11 with great commercialization potential.
Taxonomic identification of the thermotolerant and fast-growing fungus Lichtheimia ramosa H71D and biochemical characterization of the thermophilic xylanase LrXynA.[Pubmed:29098440]
AMB Express. 2017 Nov 2;7(1):194.
The zygomycete fungus Lichtheimia ramosa H71D, isolated from sugarcane bagasse compost, was identified by applying phylogenetic analysis based on the DNA sequence of the Internal Transcribed Spacer (ITS), and subsequent secondary structure analysis of ITS2. L. ramosa H71D was able to grow over a wide range of temperatures (25-45 degrees C), manifesting optimal growth at 37 degrees C. A 64 kDa xylanase (named LrXynA) was purified from the culture supernatant of L. ramosa H71D grown on 2% carboxymethylcellulose (CMC), as the only carbon source. LrXynA displayed optimal activity at pH 6 and temperature of 65 degrees C. The enzyme retained more than 50% of its maximal activity over a broad range of pH values (4.5-7.5). Enzyme half-life (t(1/2)) times at 55, 65 and 75 degrees C were 80, 25, and 8 min, respectively. LrXynA showed higher affinity (k M of 2.87 mg/mL) and catalytic efficiency (k cat /k M of 0.651 mg s/mL) towards Beechwood xylan in comparison to other substrates such as Birchwood xylan, Oat-spelt xylan, CMC, Avicel and Solka floc. The predominant final products from LrXynA-mediated hydrolysis of Beechwood xylan were xylobiose and Xylotriose, suggesting that the enzyme is an endo-beta-1,4 xylanase. Scanning electron microscopy (SEM) imaging of sugar cane bagasse (SCB) treated with LrXynA, alone or in combination with commercial cellulases, showed a positive effect on the hydrolysis of SCB. To our knowledge, this is the first report focusing on the biochemical and functional characterization of an endo-beta-1,4 xylanase from the thermotolerant and fast-growing fungus Lichtheimia ramosa.
Biochemical and biophysical characterization of novel GH10 xylanase prospected from a sugar cane bagasse compost-derived microbial consortia.[Pubmed:29274424]
Int J Biol Macromol. 2018 Apr 1;109:560-568.
Environmental issues are promoting the development of innovative technologies for the production of renewable energy and "green products" from plant biomass residues. These technologies rely on the conversion of the plant cell wall (PCW) polysaccharides into simple sugars, which involve synergistic activities of different PCW degrading enzymes, including xylanases; these are widely applied in food and feed sectors, paper and textile industries, among others. We cloned, expressed and biochemically characterized a novel xylanase (Xyn10) from the GH10 identified in a metatranscriptome of compost-derived microbial consortia and determined its low-resolution SAXS molecular envelope in solution. Our results reveal that Xyn10 is a monomeric flexible globular enzyme, with high stability with a broad pH range from 4 to 10 and optimal activity conditions at pH 7 and 40 degrees C. Only 10% of activity loss was observed after the enzyme was incubated for 30h at 40 degrees C with a pH ranging from 5 to 10. Moreover, Xyn10 maintained 100% of its initial activity after incubation for 120h at 40 degrees C and 51% after incubation for 24h at 50 degrees C (pH=7.0). Xyn10 shows endocatalytic activity towards xylan and arabinoxylan, liberating xylose, xylobiose, 1,2-alpha-d-methylglucuronic acid decorated Xylotriose, and 1,3-alpha-l-arabinofuranose decorated xylobiose and Xylotriose oligosaccharides.


