A-317491P2X3 and P2X2/3 receptor antagonist CAS# 475205-49-3 |
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475205-49-3 | SDF | Download SDF |
| PubChem ID | 9829395 | Appearance | Powder |
| Formula | C33H27NO8 | M.Wt | 565.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 47 mg/mL (83.10 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
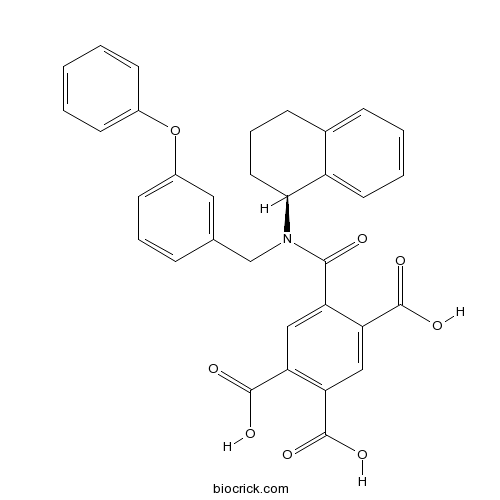
| Chemical Name | 5-[(3-phenoxyphenyl)methyl-[(1S)-1,2,3,4-tetrahydronaphthalen-1-yl]carbamoyl]benzene-1,2,4-tricarboxylic acid | ||
| SMILES | C1CC(C2=CC=CC=C2C1)N(CC3=CC(=CC=C3)OC4=CC=CC=C4)C(=O)C5=CC(=C(C=C5C(=O)O)C(=O)O)C(=O)O | ||
| Standard InChIKey | VQGBOYBIENNKMI-LJAQVGFWSA-N | ||
| Standard InChI | InChI=1S/C33H27NO8/c35-30(25-17-27(32(38)39)28(33(40)41)18-26(25)31(36)37)34(29-15-7-10-21-9-4-5-14-24(21)29)19-20-8-6-13-23(16-20)42-22-11-2-1-3-12-22/h1-6,8-9,11-14,16-18,29H,7,10,15,19H2,(H,36,37)(H,38,39)(H,40,41)/t29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A-317491 is a non-nucleotide P2X3 and P2X2/3 receptor antagonist, which inhibits calcium flux mediated by the receptors.
IC50 value:
Target: P2X2/3
It is known that P2X3 and P2X2/3 receptors stimulate the pronociceptive effects of ATP upon activation. Studies indicate that the P2X3 receptor is implicated in both neuropathic and inflammatory pain. P2X3 receptor is a promising target for therapeutic intervention in cancer patients for pain management. References: | |||||

A-317491 Dilution Calculator

A-317491 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7681 mL | 8.8406 mL | 17.6813 mL | 35.3626 mL | 44.2032 mL |
| 5 mM | 0.3536 mL | 1.7681 mL | 3.5363 mL | 7.0725 mL | 8.8406 mL |
| 10 mM | 0.1768 mL | 0.8841 mL | 1.7681 mL | 3.5363 mL | 4.4203 mL |
| 50 mM | 0.0354 mL | 0.1768 mL | 0.3536 mL | 0.7073 mL | 0.8841 mL |
| 100 mM | 0.0177 mL | 0.0884 mL | 0.1768 mL | 0.3536 mL | 0.442 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A-317491 is a high-affinity and selective antagonist of P2X2/3 and P2X3 receptors with Ki values of 9 and 22 nM, respectively for human P2X2/3 and P2X3 [1].
The P2X3 receptor is an ATP-sensitive ligand-gated ion channel expressed on sensory afferent neurons. When it combines with the P2X2 receptor, they form as a heteromeric receptor P2X2/3. Unlike the P2X2 receptor, P2X2/3 can be activated by low concentration of α, β-me ATP which was the agonist of P2X3. As an ion channel, the P2X3 receptor plays roles in the pain signaling propagation. A-317491 is the first non-nucleotide antagonist of P2X2/3 and P2X3 receptors with high selectivity. It showed anti-nociceptive in animal models of neuropathic pain and chronic inflammatory [2].
In cell membrane of 1321N1 human astrocytoma cells stably transfected with individual human P2X2 and P2X3 receptors, 3 nM of A-317491 showed 60% of total binding and the binding could be enhanced by the addition of CaCl2. Besides that, the binding was found to be reversible. In rat DRG neurons, A-317491 blocked DRG currents dose-dependently with IC50 value of 15 nM. A-317491 is highly selective against P2X2/3 and P2X3, it showed less potent effects against other P2X and the P2Y2 receptors such as P2X1 (Ki value of 2.5 μM) and P2X2 (Ki value of 4.1 μM) [2 and 3].
In rat model with CFA-induced thermal hyperalgesia, intrathecal administration of A-317491 at doses of 30 and 100 nM both showed significant anti-hyperalgesia effects. When delivered as intraplantar administration, A-317491 showed notably anti-hyperalgesia effects only at dose of 300 nM. In carrageenan-treated rats, intrathecal administration of A-317491 at doses of 30 and 100 nM also exerted anti-hyperalgesia effects. In both CCI and L5-L6 models of neuropathic allodynia, intrathecal administration of A-317491 at doses of 10 and 30 nM resulted in significant withdrawal responses to von Frey hair stimulation [4].
References:
[1] Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17179-84.
[2] Neelands TR, Burgard EC, Uchic ME, McDonald HA, Niforatos W, Faltynek CR, Lynch KJ, Jarvis MF. 2', 3'-O-(2,4,6,trinitrophenyl)-ATP and A-317491 are competitive antagonists at a slowly desensitizing chimeric human P2X3 receptor. Br J Pharmacol. 2003 Sep;140(1):202-10. Epub 2003 Jul 29.
[3] Jarvis MF, Bianchi B, Uchic JT, Cartmell J, Lee CH, Williams M, Faltynek C. [3H]A-317491, a novel high-affinity non-nucleotide antagonist that specifically labels human P2X2/3 and P2X3 receptors. J Pharmacol Exp Ther. 2004 Jul;310(1):407-16.
[4] McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003 Dec;140(8):1381-8.
- 2-Methylthioadenosine diphosphate trisodium salt
Catalog No.:BCC5794
CAS No.:475193-31-8
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- ZSTK474
Catalog No.:BCC3657
CAS No.:475110-96-4
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- NS 304
Catalog No.:BCC7661
CAS No.:475086-01-2
- Nuciferine
Catalog No.:BCN1223
CAS No.:475-83-2
- Glaucine
Catalog No.:BCN2550
CAS No.:475-81-0
- Aristolochic acid B
Catalog No.:BCN6263
CAS No.:475-80-9
- Liriodenine
Catalog No.:BCN5532
CAS No.:475-75-2
- (+)-Isocorynoline
Catalog No.:BCN2361
CAS No.:475-67-2
- H-N-Me-Pro-OH
Catalog No.:BCC3351
CAS No.:475-11-6
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Xylotriose
Catalog No.:BCN8428
CAS No.:47592-59-6
- Lycorine
Catalog No.:BCN2409
CAS No.:476-28-8
- Chelidonine
Catalog No.:BCN2463
CAS No.:476-32-4
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Therapeutic effects of the putative P2X3/P2X2/3 antagonist A-317491 on cyclophosphamide-induced cystitis in rats.[Pubmed:17917716]
Naunyn Schmiedebergs Arch Pharmacol. 2008 Jun;377(4-6):483-90.
It is suggested that ATP and purinergic P2X receptors are involved in overactive bladder. In this study, we investigated the effect of the recently developed P2X3 and P2X2/3 receptor antagonist A-317491 on cyclophosphamide (CYP)-induced cystitis to determine whether a P2X receptor antagonist could be beneficial for the treatment of bladder overactivity induced by CYP. Female Sprague-Dawley (SD) rats were given 150 mg/kg CYP (i.p.). When the micturition activity was observed for 24 h in a conscious and unrestrained condition, CYP-treated rats exhibited increased urinary frequency. Two days after CYP injection, cystometry was performed in conscious rats, in which the bladder was continuously infused with saline (5 ml/h). In CYP-treated rats, non-voiding contractions were interposed between micturitions, suggestive of hyper-reflexia. Intravenous administration of A-317491 (20 or 50 mg/kg) or pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid) tetrasodium (PPADS; a nonselective purinergic receptor antagonist, 10 mg/kg) prolonged the interval of voiding contraction and reduced the non-voiding contractions. On the other hand, oxybutynin (1 mg/kg), a muscarinic receptor antagonist, did not affect the frequency of non-voiding or voiding contractions in CYP-treated rats. A-317491 at the higher dose decreased the amplitude of voiding contractions, but increased the micturition volume. The residual urine in the bladder increased after treatment with CYP; A-317491 and PPADS reduced this, whereas oxybutynin had no effect. These data suggest that A-317491 is effective at improving the signs of CYP-induced cystitis and that the P2X3 or P2X2/3 receptor pathway is involved in bladder overactivity observed during CYP-induced cystitis.
A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats.[Pubmed:15507220]
Eur J Pharmacol. 2004 Nov 3;504(1-2):45-53.
The effect of A-317491 (5-([(3-Phenoxybenzyl)[(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbonyl)-1,2 ,4-benzenetricarboxylic acid), a recently described selective P2X3 and P2X(2/3) receptor antagonist, on inflammatory mechanical hyperalgesia was examined. In the rat Freund's complete adjuvant model of inflammatory pain, s.c. administration of A-317491 dose-dependently reversed mechanical hyperalgesia. Maximum percent reversal (72%) was seen 3 h after administration at 10 mg/kg. Substantial plasma concentrations were measured for A-317491 after s.c. dosing 3, 10 and 30 mg/kg. However, the brain-to-plasma concentration ratio, determined 1 h after a 10 mg/kg s.c. dose, indicated limited penetration of A-317491 into the central nervous system. As revealed by neural activity recorded from single C-fiber nociceptive afferent in a Freund's complete adjuvant-inflamed rat skin-nerve preparation, topical application of A-317491 completely blocked afferent activation and mechanical sensitization induced by alpha,beta-methylene ATP, a P2X agonist. These results suggest that A-317491 is a peripherally acting P2X blocker. Its efficacy demonstrates the importance of peripheral P2X3/P2X(2/3) receptors in mediating ATP-associated mechanical hyperalgesia following inflammation, confirming previous suggestions of a significant role for P2X(2/3).
Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice.[Pubmed:22634164]
Eur J Pharmacol. 2012 Aug 5;688(1-3):27-34.
The purinergic P2X3 and P2X2/3 receptors are in the peripheral nervous system almost exclusively confined to afferent sensory neurons, where they are found both at peripheral and central synapses. The P2X3 receptor is implicated in both neuropathic and inflammatory pain. However, the role of the P2X3 receptor in chronic cancer-induced bone pain is less known. Here we investigated the effect of systemic acute and chronic administration of the selective P2X3, P2X2/3 receptor antagonist (5-[[[(3-Phenoxyphenyl)methyl][(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbo nyl]-1,2,4-benzenetricarboxylic acid sodium salt hydrate) (A-317491) in a murine model of cancer-induced bone pain. Chronic administration of A-317491 (30 mumol/kgs.c., b.i.d.) resulted in a transient attenuation of pain related behaviours in the early stage of the bone cancer model, but had no effect in the late and more progressed stage of bone cancer. Also, acute administration of A-317491 (100 mumol/kgs.c.) had no effect in the progressed stage of the bone cancer pain model. Thus, systemically administered A-317491 did not demonstrate a robust effect in the present mouse model of cancer-induced bone pain.
Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states.[Pubmed:25041872]
J Neurosci Res. 2014 Dec;92(12):1703-13.
P2X is a family of ligand-gated ion channels that act through adenosine ATP. The P2X3 receptor plays a key role in the transmission of neuropathic pain at peripheral and spinal sites. Electroacupuncture (EA) has been used to treat neuropathic pain effectively. To determine the role of EA in neuropathic pain mediated through the P2X3 receptor in dorsal root ganglion neurons and the spinal cord, a chronic constriction injury (CCI) model was used. Sprague-Dawley rats were divided into four groups: sham CCI, CCI, CCI plus contralateral EA, and CCI plus ipsilateral EA. The mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were recorded. Furthermore, the expression of the P2X3 receptor was evaluated through Western blotting and immunofluorescence. The effects of EA and A-317491 were investigated through the whole-cell patch-clamp method and intrathecal administration. Our results show that the MWT and TWL of EA groups were higher than those in the CCI group, whereas the expression of the P2X3 receptor was lower than that in the CCI group. However, no significant difference was detected between the two EA groups. EA depressed the currents created by ATP and the upregulation of the P2X3 receptor in CCI rats. Additionally, EA was more potent in reducing mechanical allodynia and thermal hyperalgesia when combined with A-317491 through intrathecal administration. These results show that both contralateral and ipsilateral EA might inhibit the primary afferent transmission of neuropathic pain induced through the P2X3 receptor. In addition, EA and A-317491 might have an additive effect in inhibiting the transmission of pain mediated by the P2X3 receptor.


