WIN 64338 hydrochloridebradykinin B2 receptor antagonist, competitive CAS# 163727-74-0 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163727-74-0 | SDF | Download SDF |
| PubChem ID | 9832172 | Appearance | Powder |
| Formula | C45H69Cl2N4OP | M.Wt | 783.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in DMSO | ||
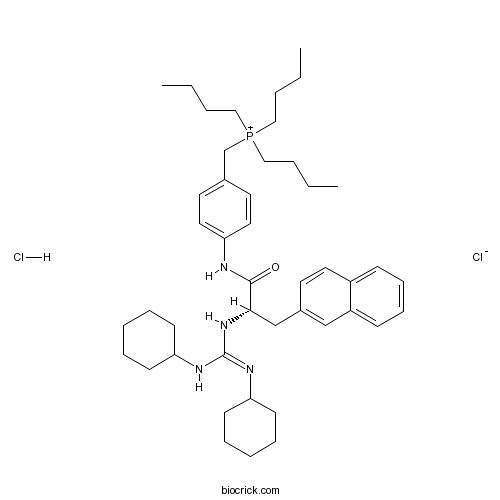
| Chemical Name | tributyl-[[4-[[(2S)-2-[(N,N'-dicyclohexylcarbamimidoyl)amino]-3-naphthalen-2-ylpropanoyl]amino]phenyl]methyl]phosphanium;chloride;hydrochloride | ||
| SMILES | CCCC[P+](CCCC)(CCCC)CC1=CC=C(C=C1)NC(=O)C(CC2=CC3=CC=CC=C3C=C2)NC(=NC4CCCCC4)NC5CCCCC5.Cl.[Cl-] | ||
| Standard InChIKey | YYJGBEZPVOUBMJ-KRFCICRISA-N | ||
| Standard InChI | InChI=1S/C45H67N4OP.2ClH/c1-4-7-30-51(31-8-5-2,32-9-6-3)35-36-25-28-42(29-26-36)46-44(50)43(34-37-24-27-38-18-16-17-19-39(38)33-37)49-45(47-40-20-12-10-13-21-40)48-41-22-14-11-15-23-41;;/h16-19,24-29,33,40-41,43H,4-15,20-23,30-32,34-35H2,1-3H3,(H2-,46,47,48,49,50);2*1H/t43-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The first potent, non-peptide, competitive bradykinin B2 receptor antagonist. In organ bath studies, WIN 64338 inhibits [3H]-bradykinin binding on guinea pig trachea with nanomolar affinity but is not active in the rabbit aorta (the classical bradykinin B1 preparation). |

WIN 64338 hydrochloride Dilution Calculator

WIN 64338 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2756 mL | 6.378 mL | 12.7559 mL | 25.5118 mL | 31.8898 mL |
| 5 mM | 0.2551 mL | 1.2756 mL | 2.5512 mL | 5.1024 mL | 6.378 mL |
| 10 mM | 0.1276 mL | 0.6378 mL | 1.2756 mL | 2.5512 mL | 3.189 mL |
| 50 mM | 0.0255 mL | 0.1276 mL | 0.2551 mL | 0.5102 mL | 0.6378 mL |
| 100 mM | 0.0128 mL | 0.0638 mL | 0.1276 mL | 0.2551 mL | 0.3189 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WIN 64338 hydrochloride is a potent and competitive antagonist of bradykinin B2 receptor [1].
Bradykinin B2 receptor is a G-protein coupled receptor for bradykinin. Bradykinin is an inflammatory nonapeptide and plays a critical role in edema, vasodilation, pain fiber stimulation and smooth muscle spasm.
WIN 64338 hydrochloride is a potent and competitive bradykinin B2 receptor antagonist. In human IMR-90 cells, WIN 64338 inhibited bradykinin binding to the bradykinin B2 receptor with Ki value of 64 nM and inhibited Ca2+ efflux stimulated by bradykinin with pA2 value of 7.1 in a competitive way. WIN 64338 inhibited guinea pig ileum contractility induced by bradykinin with pA2 value of 8.2 and also inhibited acetyicholine-induced contractility [1]. In iris sphincter isolated from rabbit, WIN 64338 (1-10 μM) inhibited contractile responses evoked by bradykinin with pKB value of 6.6 [2]. In guinea-pig tracheal smooth muscle cells, WIN 64338 inhibited inositol phosphate formation induced by bradykinin [3].
In guinea-pigs, WIN 64338 (30 nM) significantly inhibited the increases in plasma extravasation induced by bradykinin via the release of tachykinins from the trigeminal nerve [2].
References:
[1]. Sawutz DG, Salvino JM, Dolle RE, et al. The nonpeptide WIN 64338 is a bradykinin B2 receptor antagonist. Proc Natl Acad Sci U S A, 1994, 91(11): 4693-4697.
[2]. Hall JM, Figini M, Butt SK, et al. Inhibition of bradykinin-evoked trigeminal nerve stimulation by the non-peptide bradykinin B2 receptor antagonist WIN 64338 in vivo and in vitro. Br J Pharmacol, 1995, 116(8): 3164-3168.
[3]. Scherrer D, Schmidlin F, Lach E, et al. Effect of WIN 64338, a B2 bradykinin receptor antagonist on guinea-pig tracheal smooth muscle cells in culture. Fundam Clin Pharmacol, 1998, 12(2): 188-193.
- Pazufloxacin mesilate
Catalog No.:BCC9114
CAS No.:163680-77-1
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Triptoquinonide
Catalog No.:BCN1724
CAS No.:163513-81-3
- Flufenamic acid
Catalog No.:BCC9162
CAS No.:530-78-9
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Stachybotrylactam
Catalog No.:BCN6967
CAS No.:163391-76-2
- Auristatin F
Catalog No.:BCC5522
CAS No.:163768-50-1
- YM 90709
Catalog No.:BCC7149
CAS No.:163769-88-8
- 7beta-Hydroxyrutaecarpine
Catalog No.:BCN6500
CAS No.:163815-35-8
- Salvianolic acid Y
Catalog No.:BCN8123
CAS No.:1638738-76-7
- Androstenediol-3-acetate
Catalog No.:BCC8829
CAS No.:1639-43-6
- 8(17),12E,14-Labdatrien-20-oic acid
Catalog No.:BCN7396
CAS No.:1639257-36-5
- 12E,14-Labdadien-20,8beta-olide
Catalog No.:BCN7395
CAS No.:1639257-37-6
- Kaempferol-3-O-(6''-O-cis-coumaryl)glucoside
Catalog No.:BCN1538
CAS No.:163956-16-9
- Longiferone B
Catalog No.:BCN7378
CAS No.:1639810-67-5
- Clerodenoside A
Catalog No.:BCN1725
CAS No.:164022-75-7
- LY 311727
Catalog No.:BCC7728
CAS No.:164083-84-5
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
Action of bradykinin in the submucosal plexus of guinea pig small intestine.[Pubmed:14718600]
J Pharmacol Exp Ther. 2004 Apr;309(1):320-7.
Intracellular recording methods with "sharp" microelectrodes were used to study actions of bradykinin (BK) on electrical behavior of morphologically identified neurons and the identification and localization of BK receptors in the submucosal plexus of guinea pig small intestine. Exposure to BK depolarized the membrane potential and elevated excitability in submucosal neurons with AH-type electrophysiological behavior and Dogiel II multipolar morphology and in neurons with S-type electrophysiological behavior and uniaxonal morphology. BK-evoked depolarizing responses were associated with increased neuronal input resistance in AH-type neurons and decreased input resistance in S-type neurons. The selective B(2) BK receptor antagonists HOE-140 (icatabant acetate) and WIN64338 [(S)-4[2-bis(cyclohexylamino)methyleneamino]-3-(2-napthalenyl)-1-oxopropylamino]b enzyl tributyl phosphonium chloride hydrochloride], but not the selective B(1) receptor antagonists des-arg(10)-HOE-140 and des-arg(9)-leu(8)-BK, suppressed the BK-evoked responses. The selective B(2) receptor agonist Kallidin, but not the selective B(1) receptor agonist des-arg(9)-BK mimicked the excitatory action of BK. Western blot analysis and reverse transcription-polymerase chain reaction confirmed the expression of B(2) receptor protein and mRNA. Binding studies with a fluorescently labeled BK(2) antagonist found expression of B(2) receptors on a majority of the ganglion cells. B(2) receptors occupied 82% of the neurons that expressed immunoreactivity for neuropeptide Y, 75% of the neurons that expressed vasoactive intestinal peptide, 84% of the neurons that expressed substance P, 71% of the neurons that expressed choline acetyltransferase, and all neurons that expressed calbindin immunoreactivity. The results suggest that the B(2) receptor mediates the excitatory action of BK on submucosal plexus neurons. Pathophysiological significance of the excitatory actions on secretomotor neurons might be stimulated mucosal secretion and the secretory diarrhea associated with intestinal inflammatory states.
Effects of WIN 64338, a nonpeptide bradykinin B2 receptor antagonist, on guinea-pig trachea.[Pubmed:7582533]
Br J Pharmacol. 1995 Aug;115(7):1127-8.
We investigated the effect of the nonpeptide bradykinin receptor antagonist, [[4-[[2-[[bis(cyclohexylamino)methylene] amino]-3-(2-naphthalenyl) 1-oxopropyl]amino]-phenyl]-tributyl, chloride, monohydrochloride (WIN 64338), on [3H]-bradykinin binding and on bradykinin-induced contraction of the guinea-pig trachea. This non peptide bradykinin receptor antagonist inhibited [3H]-bradykinin binding with a nanomolar range of affinity, Ki = 50.9 +/- 19 nM and inhibited bradykinin-induced contraction in a non-competitive manner with a KB value of 6.43 10(-8) +/- 2.34 10(-8) M.
Effects of peptide and nonpeptide antagonists of bradykinin B2 receptors on the venoconstrictor action of bradykinin.[Pubmed:8014858]
J Pharmacol Exp Ther. 1994 Jun;269(3):1136-43.
The isolated rabbit jugular and human umbilical veins respond to bradykinin (BK) by contractions that are mediated by the BK B2 type receptors. In this report, the pharmacology of recently developed BK B2 receptor antagonists is assessed by using these preparations. The nonpeptide kinin antagonist WIN 64338 (phosphonium, [[4-[[2-[[bis(cyclohexylamino)methylene]amino]- 3-(2-naphthalenyl)-1-oxopropyl]amino]phenyl]methyl]tributyl chloride monohydrochloride) demonstrates competitive and surmountable antagonism of BK in both the jugular and the umbilical veins (pA2 values of 6.14 and 5.99, respectively). WIN 64338 shows selectivity in its antagonist action as it does not inhibit the effect of various other contractile agents in either of the preparations. HOE-140 (D-Arg[hydroxyproline3,beta-thienylalanine5, D-Tic7, octahydroindol-2-yl-carbonyl residue8]-BK), a "second generation" peptide antagonist of BK, behaves as an insurmountable and irreversible antagonist in the rabbit jugular vein, but appears to be competitive in the umbilical vein (pA2 = 8.2). In the jugular vein, [L-Tic7]HOE-140 is an insurmountable antagonist about 2000-fold less potent than HOE-140; the L-Tic7 isomer demonstrates no significant antagonist activity on the umbilical vein at 30 microM. This study confirms that WIN 64338 behaves as a competitive and selective kinin antagonist of the BK B2 type receptors. The pharmacological profile of the L-Tic7 analog of HOE-140 may provide useful information in discerning the molecular interaction of noncompetitive BK antagonists with their receptors.


