Flufenamic acidCAS# 530-78-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 530-78-9 | SDF | Download SDF |
| PubChem ID | 3371 | Appearance | Powder |
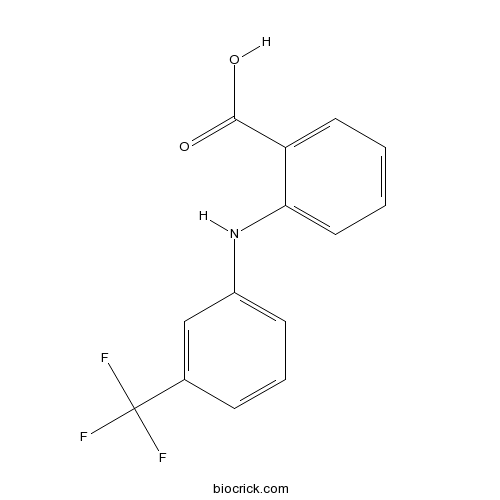
| Formula | C14H10F3N2O2 | M.Wt | 295.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Fluphenamic acid;Nichisedan | ||
| Solubility | DMSO : 100 mg/mL (355.58 mM; Need ultrasonic) | ||
| Chemical Name | 2-[3-(trifluoromethyl)anilino]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)C(=O)O)NC2=CC=CC(=C2)C(F)(F)F | ||
| Standard InChIKey | LPEPZBJOKDYZAD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10F3NO2/c15-14(16,17)9-4-3-5-10(8-9)18-12-7-2-1-6-11(12)13(19)20/h1-8,18H,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Flufenamic acid Dilution Calculator

Flufenamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3875 mL | 16.9377 mL | 33.8753 mL | 67.7507 mL | 84.6883 mL |
| 5 mM | 0.6775 mL | 3.3875 mL | 6.7751 mL | 13.5501 mL | 16.9377 mL |
| 10 mM | 0.3388 mL | 1.6938 mL | 3.3875 mL | 6.7751 mL | 8.4688 mL |
| 50 mM | 0.0678 mL | 0.3388 mL | 0.6775 mL | 1.355 mL | 1.6938 mL |
| 100 mM | 0.0339 mL | 0.1694 mL | 0.3388 mL | 0.6775 mL | 0.8469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Stachybotrylactam
Catalog No.:BCN6967
CAS No.:163391-76-2
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Bethoxazin
Catalog No.:BCC5471
CAS No.:163269-30-5
- Sitafloxacin Hydrate
Catalog No.:BCC4959
CAS No.:163253-35-8
- Clevudine
Catalog No.:BCC4770
CAS No.:163252-36-6
- 680C91
Catalog No.:BCC6158
CAS No.:163239-22-3
- Ezetimibe
Catalog No.:BCN2180
CAS No.:163222-33-1
- Cimifugin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN7853
CAS No.:1632110-81-6
- (-)-[3R,4S]-Chromanol 293B
Catalog No.:BCC7080
CAS No.:163163-24-4
- Chromanol 293B
Catalog No.:BCC7055
CAS No.:163163-23-3
- Triptoquinonide
Catalog No.:BCN1724
CAS No.:163513-81-3
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
- Pazufloxacin mesilate
Catalog No.:BCC9114
CAS No.:163680-77-1
- WIN 64338 hydrochloride
Catalog No.:BCC6914
CAS No.:163727-74-0
- Auristatin F
Catalog No.:BCC5522
CAS No.:163768-50-1
- YM 90709
Catalog No.:BCC7149
CAS No.:163769-88-8
- 7beta-Hydroxyrutaecarpine
Catalog No.:BCN6500
CAS No.:163815-35-8
Flufenamic acid inhibits secondary hemorrhage and BSCB disruption after spinal cord injury.[Pubmed:30128046]
Theranostics. 2018 Jul 30;8(15):4181-4198.
Acute spinal cord injury (SCI) induces secondary hemorrhage and initial blood-spinal cord barrier (BSCB) disruption. The transient receptor potential melastatin 4 (Trpm4) together with sulfonylurea receptor 1 (Sur1) forms the Sur1-Trpm4 channel complex. The up-regulation of Sur1-Trpm4 after injury plays a crucial role in secondary hemorrhage, which is the most destructive mechanism in secondary injuries of the central nervous system (CNS). The matrix metalloprotease (MMP)-mediated disruption of the BSCB leads to an inflammatory response, neurotoxin production and neuronal cell apoptosis. Thus, preventing secondary hemorrhage and BSCB disruption should be an important goal of therapeutic interventions in SCI. Methods: Using a moderate contusion injury model at T10 of the spinal cord, Flufenamic acid (FFA) was injected intraperitoneally 1 h after SCI and then continuously once per day for one week. Results: Trpm4 expression is highly up-regulated in capillaries 1 d after SCI. Treatment with Flufenamic acid (FFA) inhibited Trpm4 expression, secondary hemorrhage, and capillary fragmentation and promoted angiogenesis. In addition, FFA significantly inhibited the expression of MMP-2 and MMP-9 at 1 d after SCI and significantly attenuated BSCB disruption at 1 d and 3 d after injury. Furthermore, we found that FFA decreased the hemorrhage- and BSCB disruption-induced activation of microglia/macrophages and was associated with smaller lesions, decreased cavity formation, better myelin preservation and less reactive gliosis. Finally, FFA protected motor neurons and improved locomotor functions after SCI. Conclusion: This study indicates that FFA improves functional recovery, in part, due to the following reasons: (1) it inhibits the expression of Trpm4 to reduce the secondary hemorrhage; and (2) it inhibits the expression of MMP-2 and MMP-9 to block BSCB disruption. Thus, the results of our study suggest that FFA may represent a potential therapeutic agent for promoting functional recovery.
Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase.[Pubmed:21765041]
J Pharmacol Exp Ther. 2011 Oct;339(1):257-66.
Flufenamic acid (FFA) is a nonsteroidal anti-inflammatory drug (NSAID). It has anti-inflammatory and antipyretic properties. In addition, it modulates multiple channel activities. The mechanisms underlying the pharmacological actions of FFA are presently unclear. Given that AMP-activated protein kinase (AMPK) has both anti-inflammatory and channel-regulating functions, we examined whether FFA induces AMPK activation. 1) Exposure of several different types of cells to FFA resulted in an elevation of AMPKalpha phosphorylation at Thr172. This effect of FFA was reproduced by functionally and structurally similar mefenamic acid, tolfenamic acid, niflumic acid, and meclofenamic acid. 2) FFA-induced activation of AMPK was largely abolished by the treatment of cells with 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester) (an intracellular Ca(2+) chelator) or depletion of extracellular Ca(2+), whereas it was mimicked by stimulation of cells with the Ca(2+) ionophore 5-(methylamino)-2-({(2R,3R,6S,8S,9R,11R)-3,9,11-trimethyl-8-[(1S)-1-methyl-2-oxo- 2-(1H-pyrrol-2-yl)ethyl]-1,7-dioxaspiro[5.5]undec-2-yl}methyl)-1,3-benzoxazole-4- carboxylic acid (A23187) or ionomycin. 3) FFA triggered a rise in intracellular Ca(2+), which was abolished by cyclosporine, a blocker of mitochondrial permeability transition pore. Cyclosporine also abolished FFA-induced activation of AMPK. 4) Inhibition of Ca(2+)/calmodulin-dependent kinase kinase beta (CaMKKbeta) with 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate (STO-609) or down-regulation of CaMKKbeta with short interfering RNA largely abrogated FFA-induced activation of AMPK. 5) FFA significantly suppressed nuclear factor-kappaB activity and inducible nitric-oxide synthase expression triggered by interleukin-1beta and tumor necrosis factor alpha. This suppression was also largely abrogated by STO-609. Taken together, we conclude that FFA induces AMPK activation through the Ca(2+)-CaMKKbeta pathway. Activation of AMPK is a presently unrecognized important mechanism underlying the pharmacological effects of FFA.
Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells.[Pubmed:19232718]
Cell Calcium. 2009 Apr;45(4):384-90.
Mutations in the cation channel TRPC6 result in a renal-specific phenotype of familial nephrotic syndrome, affecting intracellular calcium ([Ca(2+)](i)) signalling in the glomerular podocyte. Tools to study native TRPC6 activity are scarce, although there has been recent success with Flufenamic acid (FFA). We confirm the specificity of FFA for TRPC6 both in an artificial expression system and in a human conditionally immortalised podocyte cell line (ciPod). Cells were loaded with fura-2AM and changes in intracellular calcium ([Ca(2+)](i)) were calculated. 200microM FFA induced an increase in [Ca(2+)](i) in HEK293 cells with native TRPC6 expression, which was enhanced by overexpression of TRPC6 and completely blocked in the absence of extracellular calcium. Expressed TRPC7 did not significantly affect the response to FFA whereas expressed TRPC3 reduced it. FFA also induced an increase ciPod in [Ca(2+)](i), which was inhibited using SKF96365 and 2-APB, but not indomethacin. In ciPod, adenovirus (Ad-v) wild type (WT) TRPC6 increased [Ca(2+)](i) activity to FFA compared to native TRPC6, whereas activity was significantly reduced with Ad-v dominant negative (DN) TRPC6. The niflumic acid (NFA) induced increase in [Ca(2+)](i) in ciPod was not affected by Ad-v TRPC6 DN, and in HEK293 cells was not affected by WT TRPC6. In conclusion, FFA activates TRPC6 [Ca(2+)](i) signalling in both ciPod and HEK293 cells independently of TRPC3 and TRPC7, and independently of properties of the fenamate family.
Diacylglycerol analogues activate second messenger-operated calcium channels exhibiting TRPC-like properties in cortical neurons.[Pubmed:19094061]
J Neurochem. 2009 Jan;108(1):126-38.
The lipid diacylglycerol (DAG) analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) was used to verify the existence of DAG-sensitive channels in cortical neurons dissociated from E13 mouse embryos. Calcium imaging experiments showed that OAG increased the cytosolic concentration of Ca(2+) ([Ca(2+)]i) in nearly 35% of the KCl-responsive cells. These Ca(2+) responses disappeared in a Ca(2+)-free medium supplemented with EGTA. Mn(2+) quench experiments showed that OAG activated Ca(2+)-conducting channels that were also permeant to Ba(2+). The OAG-induced Ca(2+) responses were unaffected by nifedipine or omega-conotoxin GVIA (Sigma-Aldrich, Saint-Quentin Fallavier, France) but blocked by 1-[beta-(3-(4-Methoxyphenyl)propoxy)-4-methoxyphenethyl]-1H-imidazole hydrochloride (SKF)-96365 and Gd(3+). Replacing Na(+) ions with N-methyl-D-glucamine diminished the amplitude of the OAG-induced Ca(2+) responses showing that the Ca(2+) entry was mediated via Na(+)-dependent and Na(+)-independent mechanisms. Experiments carried out with the fluorescent Na(+) indicator CoroNa Green showed that OAG elevated [Na(+)]i. Like OAG, the DAG lipase inhibitor RHC80267 increased [Ca(2+)]i but not the protein kinase C activator phorbol 12-myristate 13-acetate. Moreover, the OAG-induced Ca(2+) responses were not regulated by protein kinase C activation or inhibition but they were augmented by Flufenamic acid which increases currents through C-type transient receptor potential protein family (TRPC) 6 channels. In addition, application of hyperforin, a specific activator of TRPC6 channels, elevated [Ca(2+)]i. Whole-cell patch-clamp recordings showed that hyperforin activated non-selective cation channels. They were blocked by SKF-96365 but potentiated by Flufenamic acid. Altogether, our data show the presence of hyperforin- and OAG-sensitive Ca(2+)-permeable channels displaying TRPC6-like properties. This is the first report revealing the existence of second messenger-operated channels in cortical neurons.
Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes.[Pubmed:1692608]
Mol Pharmacol. 1990 May;37(5):720-4.
The effects of niflumic acid and Flufenamic acid, two nonsteroidal anti-inflammatory agents known to block anion transport in red blood cells, on Ca2(+)-activated Cl- currents (ICl(Ca)) in Xenopus oocytes were examined. Both compounds reversibly inhibited ICl(Ca), elicited in response to depolarizing voltage steps, in a dose-dependent manner, with no effect on the shape of the current-voltage curve. The apparent inhibition constant for niflumic acid was 17 microM, whereas that for Flufenamic acid was 28 microM. Niflumic acid also inhibited ICl(Ca) elicited by bath application of Ca2+ to oocytes permeabilized using the Ca2+ ionophore A23187, demonstrating that the inhibition of ICl(Ca) is due to a direct interaction with the Cl- channel, rather than by interference with Ca2+ entry through voltage-dependent Ca2+ channels. In addition to their use in the elimination of ICl(Ca) as a possible source of artifact when Xenopus oocytes are used as an expression system for exogenous ion channels and receptors, it is expected that these two compounds will find use as potent anion channel blockers.


