TriptoquinonideCAS# 163513-81-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163513-81-3 | SDF | Download SDF |
| PubChem ID | 15762010 | Appearance | Powder |
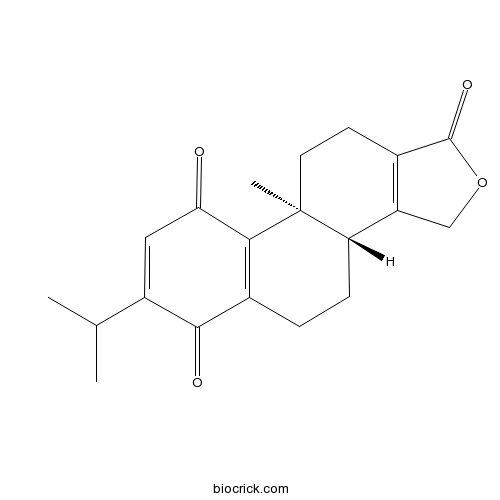
| Formula | C20H22O4 | M.Wt | 326.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3bR,9bS)-9b-methyl-7-propan-2-yl-3,3b,4,5,10,11-hexahydronaphtho[2,1-e][2]benzofuran-1,6,9-trione | ||
| SMILES | CC(C)C1=CC(=O)C2=C(C1=O)CCC3C2(CCC4=C3COC4=O)C | ||
| Standard InChIKey | SZTABFBXCBBJRR-YWZLYKJASA-N | ||
| Standard InChI | InChI=1S/C20H22O4/c1-10(2)13-8-16(21)17-12(18(13)22)4-5-15-14-9-24-19(23)11(14)6-7-20(15,17)3/h8,10,15H,4-7,9H2,1-3H3/t15-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triptoquinonide is a natural product from Tripterygium wilfordii. |
| Structure Identification | J Org Chem. 2000 Apr 7;65(7):2208-17.Enantioselective total synthesis of (-)-triptolide, (-)-triptonide, (+)-triptophenolide, and (+)-triptoquinonide.[Pubmed: 10774048]The first enantioselective total synthesis of (-)-triptolide (1), (-)-triptonide (2), (+)-triptophenolide (3), and (+)-Triptoquinonide (4) was completed. |

Triptoquinonide Dilution Calculator

Triptoquinonide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0637 mL | 15.3186 mL | 30.6373 mL | 61.2745 mL | 76.5931 mL |
| 5 mM | 0.6127 mL | 3.0637 mL | 6.1275 mL | 12.2549 mL | 15.3186 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0637 mL | 6.1275 mL | 7.6593 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6127 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6127 mL | 0.7659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Flufenamic acid
Catalog No.:BCC9162
CAS No.:530-78-9
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Stachybotrylactam
Catalog No.:BCN6967
CAS No.:163391-76-2
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Bethoxazin
Catalog No.:BCC5471
CAS No.:163269-30-5
- Sitafloxacin Hydrate
Catalog No.:BCC4959
CAS No.:163253-35-8
- Clevudine
Catalog No.:BCC4770
CAS No.:163252-36-6
- 680C91
Catalog No.:BCC6158
CAS No.:163239-22-3
- Ezetimibe
Catalog No.:BCN2180
CAS No.:163222-33-1
- Cimifugin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN7853
CAS No.:1632110-81-6
- (-)-[3R,4S]-Chromanol 293B
Catalog No.:BCC7080
CAS No.:163163-24-4
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
- Pazufloxacin mesilate
Catalog No.:BCC9114
CAS No.:163680-77-1
- WIN 64338 hydrochloride
Catalog No.:BCC6914
CAS No.:163727-74-0
- Auristatin F
Catalog No.:BCC5522
CAS No.:163768-50-1
- YM 90709
Catalog No.:BCC7149
CAS No.:163769-88-8
- 7beta-Hydroxyrutaecarpine
Catalog No.:BCN6500
CAS No.:163815-35-8
- Salvianolic acid Y
Catalog No.:BCN8123
CAS No.:1638738-76-7
Enantioselective total synthesis of (-)-triptolide, (-)-triptonide, (+)-triptophenolide, and (+)-triptoquinonide.[Pubmed:10774048]
J Org Chem. 2000 Apr 7;65(7):2208-17.
The first enantioselective total synthesis of (-)-triptolide (1), (-)-triptonide (2), (+)-triptophenolide (3), and (+)-Triptoquinonide (4) was completed. The key step involves lanthanide triflate-catalyzed oxidative radical cyclization of (+)-8-phenylmenthyl ester 30 mediated by Mn(OAc)3, providing intermediate 31 with good chemical yield (77%) and excellent diastereoselectivity (dr 38:1). (+)-Triptophenolide methyl ether (5) was then prepared in > 99% enantiomeric excess (> 99% ee), and readily converted to natural products 1-4. In addition, transition state models were proposed to explain the opposite chiral induction observed in the oxidative radical cyclization reactions of chiral beta-keto esters 17 (without an alpha-substituent) and 17a (with an alpha-chloro substituent).


