6''-O-MalonylgenistinCAS# 51011-05-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

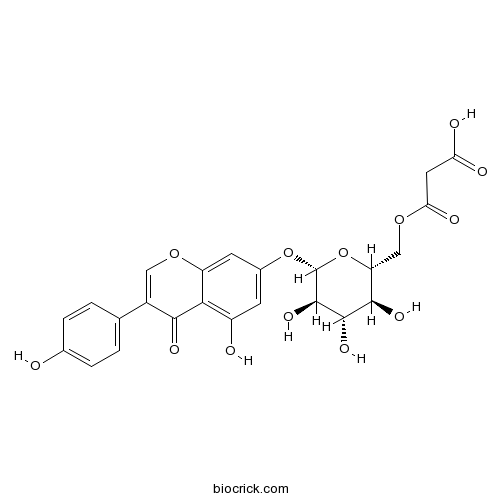
| Cas No. | 51011-05-3 | SDF | Download SDF |
| PubChem ID | 15934091 | Appearance | Powder |
| Formula | C24H22O13 | M.Wt | 518.42 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Malonylgenistin; Genistin malonate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-oxo-3-[[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxan-2-yl]methoxy]propanoic acid | ||
| SMILES | C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)OC4C(C(C(C(O4)COC(=O)CC(=O)O)O)O)O)O | ||
| Standard InChIKey | FRAUJUKWSKMNJY-RSEYPYQYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 6''-O-Malonylgenistin possesses a hepatoprotective activity. |
| In vitro | Isoflavone content and its potential contribution to the antihypertensive activity in soybean Angiotensin I converting enzyme inhibitory peptides.[Pubmed: 18921974]J Agric Food Chem. 2008 Nov 12;56(21):9899-904.A soybean angiotensin I converting enzyme (ACE) inhibitory peptide fraction was reported to have antihypertensive activity in a rat study. The purpose of the present study was to examine if the presence of isoflavones in the soybean ACE inhibitory peptide fraction may contribute to the blood-pressure-lowering property.
Isoflavonoid composition of a callus culture of the relict tree Maackia amurensis Rupr. et Maxim.[Pubmed: 18671403]J Agric Food Chem. 2008 Aug 27;56(16):7023-31.Isoflavonoids, an interesting and restricted group of secondary metabolites of legumes, exhibit estrogenic, antiangiogenic, and anticancer activities and are now popular as dietary supplements. Plant cell cultures that possess an increased ability to synthesize these metabolites were examined.
|

6''-O-Malonylgenistin Dilution Calculator

6''-O-Malonylgenistin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9289 mL | 9.6447 mL | 19.2894 mL | 38.5788 mL | 48.2234 mL |
| 5 mM | 0.3858 mL | 1.9289 mL | 3.8579 mL | 7.7158 mL | 9.6447 mL |
| 10 mM | 0.1929 mL | 0.9645 mL | 1.9289 mL | 3.8579 mL | 4.8223 mL |
| 50 mM | 0.0386 mL | 0.1929 mL | 0.3858 mL | 0.7716 mL | 0.9645 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1929 mL | 0.3858 mL | 0.4822 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
6''-O-Malonylgenistin(Malonylgenistin) is an isoflavone derivative.
References:
[1]. Fedoreyev SA, et al. Isoflavonoid composition of a callus culture of the relict tree Maackia amurensis Rupr. et Maxim. J Agric Food Chem. 2008 Aug 27;56(16):7023-31.
[2]. Wu J, et al. Isoflavone content and its potential contribution to the antihypertensive activity in soybean Angiotensin I converting enzyme inhibitory peptides. J Agric Food Chem. 2008 Nov 12;56(21):9899-904.
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- (2-Acetoxyethyl)trimethylammonium
Catalog No.:BCN1743
CAS No.:51-84-3
- Carbamoylcholine chloride
Catalog No.:BCC7492
CAS No.:51-83-2
- Tyramine
Catalog No.:BCN6776
CAS No.:51-67-2
- 4'-Methoxyacetanilide
Catalog No.:BCC8711
CAS No.:51-66-1
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
Isoflavone content and its potential contribution to the antihypertensive activity in soybean Angiotensin I converting enzyme inhibitory peptides.[Pubmed:18921974]
J Agric Food Chem. 2008 Nov 12;56(21):9899-904.
A soybean angiotensin I converting enzyme (ACE) inhibitory peptide fraction was reported to have antihypertensive activity in a rat study. The purpose of the present study was to examine if the presence of isoflavones in the soybean ACE inhibitory peptide fraction may contribute to the blood-pressure-lowering property. The isoflavone concentration in soybean samples was analyzed on a C 18 reverse-phase column using a two-step gradient solvent system. Producing soybean hydrolysate led to a nearly 40% loss of isoflavones compared with the original soybean flour, but the isoflavone composition did not change and the dominant isoflavone chemicals remained as 6''-O-malonylgenistin and 6''-O-malonyldaidzin. Ion exchange chromatography affected significantly both the content and the composition of the isoflavones. The dominant isoflavones in the ion-exchanged fraction were aglycones and nonacylated isoflavones, accounting for 95.8% of the total amount of 987.7 microg/g. It was calculated that the isoflavone content in the soybean ACE inhibitory peptide fraction was 25 times less than the minimal effective isoflavone dose reported. In vitro study also showed that adding isoflavones into both soybean flour hydrolysate and soybean ACE inhibitory peptide samples to a concentration of as high as 31.5% (w/w) did not affect ACE inhibitory activity (IC 50 values). The findings along with previously published results indicated that the contribution of isoflavones in soybean ACE inhibitory peptide fraction to the blood-pressure-lowering property in a short-term feeding study might be negligible.
Isoflavonoid composition of a callus culture of the relict tree Maackia amurensis Rupr. et Maxim.[Pubmed:18671403]
J Agric Food Chem. 2008 Aug 27;56(16):7023-31.
Isoflavonoids, an interesting and restricted group of secondary metabolites of legumes, exhibit estrogenic, antiangiogenic, and anticancer activities and are now popular as dietary supplements. Plant cell cultures that possess an increased ability to synthesize these metabolites were examined. During the investigation, cell cultures of the Far Eastern relict tree Maackia amurensis (Leguminosae) were established. A selection of seed-derived cell aggregates yielded the callus line designated A-18. This culture produces 20 isoflavonoids, namely, the isoflavones genistein, daidzein, formononetin, calycosin, derrone, and pseudobaptigenin and their glycosylated conjugates genistin, 6''-O-malonylgenistin, ononin, 6''-O-malonylononin, daidzin, 3'-methoxydaidzin, 4'-O-beta-D-glucopyranosyldaidzin, 4'-O-beta-D-glucopyranosylgenistin, and 7-O-beta-D-glucopyranosylcalycosin; the pterocarpans maackiain and medicarpin and their glycosylated conjugates 6'-O-malonyl-3-O-beta-D-glucopyranosylmaackiain and 6'-O-malonyl-3-O-beta-D-glucopyranosylmedicarpin; and the new pterocarpan glucoside 6'-O-malonyl-3-O-beta-D-glucopyranosyl-6,6a-dehydromaackiain. These isoflavonoids, possessing a hepatoprotective activity, were stably produced by the A-18 calli for prolonged periods of observation.


