LomitapideMTP inhibitor, orally active CAS# 182431-12-5 |
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 182431-12-5 | SDF | Download SDF |
| PubChem ID | 9853053 | Appearance | Powder |
| Formula | C39H37F6N3O2 | M.Wt | 693.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AEGR-733; BMS-201038 | ||
| Solubility | DMSO : ≥ 100 mg/mL (144.15 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
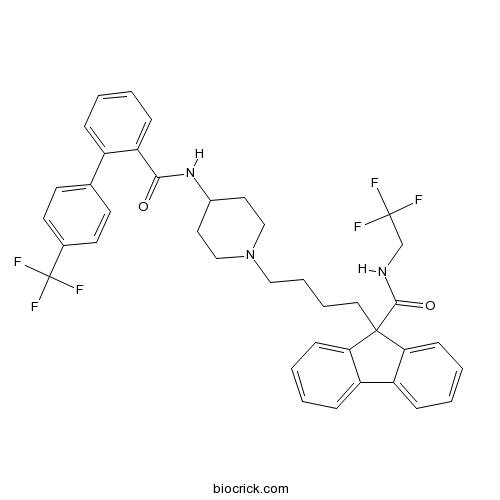
| Chemical Name | N-(2,2,2-trifluoroethyl)-9-[4-[4-[[2-[4-(trifluoromethyl)phenyl]benzoyl]amino]piperidin-1-yl]butyl]fluorene-9-carboxamide | ||
| SMILES | C1CN(CCC1NC(=O)C2=CC=CC=C2C3=CC=C(C=C3)C(F)(F)F)CCCCC4(C5=CC=CC=C5C6=CC=CC=C64)C(=O)NCC(F)(F)F | ||
| Standard InChIKey | MBBCVAKAJPKAKM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C39H37F6N3O2/c40-38(41,42)25-46-36(50)37(33-13-5-3-10-30(33)31-11-4-6-14-34(31)37)21-7-8-22-48-23-19-28(20-24-48)47-35(49)32-12-2-1-9-29(32)26-15-17-27(18-16-26)39(43,44)45/h1-6,9-18,28H,7-8,19-25H2,(H,46,50)(H,47,49) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lomitapide (AEGR-733; BMS-201038) is a potent inhibitor of microsomal triglyceride-transfer protein (MTP) with an IC50 of 8 nM in vitro.In Vitro:Lomitapide is an oral microsomal triglyceride transfer protein (MTP) inhibitor indicated for the treatment of patients with HoFH, a rare form of hypercholesterolemia that can lead to premature atherosclerotic disease. Lomitapide undergoes hepatic metabolism via cytochrome P-450 (CYP) isoenzyme 3A4 and interacts with CYP3A4 substrates including atorvastatin and simvastatin[2].In Vivo:The use of lomitapide alone or in combination with other lipid-lowering modalities reduces plasma concentrations of low density lipoprotein cholesterol (LDL-C) by a mean of more than 50%. Lomitapide is associated with significant gastrointestinal adverse effects and increases in hepatic fat levels. The bioavailability of the 50-mg lomitapide capsule is 7.1%. The mean half-life of lomitapide is 39.7 hours[2]. Single-dose administration of lomitapide is shown to reduce serum triglycerides by 35% and 47% at 0.3- and 1-mg/kg doses, respectively. Multiple-dose treatment with lomitapide also results in dose dependent decrease in triglycerides (71%–87%), nonesterified fattyacids(33%–40%), and LDL-C(26-29%)[3]. References: | |||||

Lomitapide Dilution Calculator

Lomitapide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4415 mL | 7.2077 mL | 14.4155 mL | 28.8309 mL | 36.0386 mL |
| 5 mM | 0.2883 mL | 1.4415 mL | 2.8831 mL | 5.7662 mL | 7.2077 mL |
| 10 mM | 0.1442 mL | 0.7208 mL | 1.4415 mL | 2.8831 mL | 3.6039 mL |
| 50 mM | 0.0288 mL | 0.1442 mL | 0.2883 mL | 0.5766 mL | 0.7208 mL |
| 100 mM | 0.0144 mL | 0.0721 mL | 0.1442 mL | 0.2883 mL | 0.3604 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lomitapide (AEGR-733, BMS-201038) is an oral inhibitor of microsomal triglyceride transfer protein [1].
Microsomal triglyceride transfer protein (MTP) plays an important role in lipoprotein assembly. The mutation of MTP can cause abetalipoproteinemia.
Lomitapide is a MTP inhibitor and used for the treatment of homozygous familial hypercholesterolemia. In patients with familial hypercholesterolemia, lomitapide reduced low-density lipoprotein cholesterol (LDL-C) by more than 50% both as monotherapy and in combination with prescribed lipid-lowering therapies [1]. Lomitapide bound to and inhibited MTP within the lumen of the endoplasmic reticulum and inhibited the synthesis of VLDL and chylomicrons, resulting in the reduction of circulating LDL-C concentrations. In human, lomitapide (50 mg) reduced apo B, LDL-C and total cholesterol concentrations in a dose dependent way. However, lomitapide caused significant gastrointestinal (GI) disturbances at the concentration of 100 mg [2].
References:
[1]. Rizzo M. Lomitapide, a microsomal triglyceride transfer protein inhibitor for the treatment of hypercholesterolemia. IDrugs, 2010, 13(2): 103-111.
[2]. Davis KA, Miyares MA. Lomitapide: A novel agent for the treatment of homozygous familial hypercholesterolemia. Am J Health Syst Pharm, 2014, 71(12): 1001-1008.
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Baldrinal
Catalog No.:BCN2667
CAS No.:18234-46-3
- Antibiotic 2158
Catalog No.:BCN1825
CAS No.:182320-34-9
- Antibiotic ZG 1494alpha
Catalog No.:BCN1850
CAS No.:182320-33-8
- Synthalin sulfate
Catalog No.:BCC6730
CAS No.:182285-12-7
- Lirioprolioside B
Catalog No.:BCN2740
CAS No.:182284-68-0
- Clausine I
Catalog No.:BCN4687
CAS No.:182261-94-5
- 3,4-seco-Olean-12-en-4-ol-3,28-dioic acid
Catalog No.:BCN7151
CAS No.:182249-69-0
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- 6-Angeloyloxyditropan-3-yl itaconate
Catalog No.:BCN1867
CAS No.:182015-05-0
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
Lomitapide for the treatment of hypertriglyceridemia.[Pubmed:27785928]
Expert Opin Investig Drugs. 2016 Dec;25(12):1457-1463.
INTRODUCTION: Severe genetic forms of hypertriglyceridemia carry a risk of life-threatening pancreatitis and lack available effective treatments. Lomitapide is a microsomal triglyceride transfer protein inhibitor currently approved for treatment of homozygous familial hypercholesterolemia that may be useful in the management of severe hypertriglyceridemia. Areas covered: Published trials of Lomitapide that reported plasma triglyceride response were reviewed, as was a case report of a patient with hypertriglyceridemia who was treated for 13 years with Lomitapide. ClinicalTrials.gov was also reviewed for any unpublished results and ongoing trials. Expert opinion: Lomitapide demonstrates effective triglyceride lowering and may be a useful treatment for patients with genetic hypertriglyceridemia and recurrent acute pancreatitis who are refractory to traditional treatment. However, long term hepatic safety may be a concern and direct clinical trial-level data are lacking for this indication.
Efficacy and Safety of Lomitapide in Hypercholesterolemia.[Pubmed:28255870]
Am J Cardiovasc Drugs. 2017 Aug;17(4):299-309.
BACKGROUND: Despite extensive use of statins, patients with hypercholesterolemia, especially homozygous familial hypercholesterolemia (HoFH), do not achieve recommended targets of low-density lipoprotein cholesterol (LDL-C). There is an urgent need for novel options that could reduce proatherogenic lipoprotein cholesterol levels. Lomitapide, a microsomal triglyceride transport protein (MTP) inhibitor, was approved three years ago as an orphan drug for the treatment of patients with HoFH. OBJECTIVE: Our aim was to systematically evaluate the efficacy and safety of Lomitapide and to provide guidance for clinicians. METHODS: We searched the PubMed, Embase, and Cochrane library databases and ClinicalTrials.gov to identify valid studies published before 31 October 2016 that included Lomitapide-treated patients who did or did not undergo lipid-lowering therapy. We assessed the quality of different studies. Data were extracted and evaluated for quality by two reviewers. RESULTS: Studies reporting Lomitapide therapy included one randomized controlled trial, three single-arm studies, and five case reports. In patients with HoFH, Lomitapide reduced levels of LDL-C, total cholesterol, apolipoprotein B, and triglycerides with or without other lipid-lowering therapy, including apheresis. In non-HoFH patients with moderate hypercholesterolemia and hypertriglyceridemia, Lomitapide also showed favorable effects on changes in LDL-C and triglycerides. However, both HoFH and non-HoFH patients experienced a reduction in high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-1 (ApoA-1). The most common adverse event was gastrointestinal disorder, and others included liver transaminase elevation and hepatic fat accumulation. Long-term use of Lomitapide was associated with an increased risk of progressing to steatohepatitis and fibrosis. CONCLUSIONS: Lomitapide improved most lipid parameters but not HDL-C or ApoA-1 in patients with HoFH and in non-HoFH patients, and gastrointestinal disorders were the most common adverse event. The possible benefits of Lomitapide should be further evaluated and viewed against its possible long-term side effects.
Efficacy and Safety of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia.[Pubmed:28154305]
J Atheroscler Thromb. 2017 Apr 3;24(4):402-411.
AIM: There is an unmet need in Japan for more optimal lipid-lowering therapy (LLT) for patients with homozygous familial hypercholesterolemia (HoFH) who respond inadequately to available drug therapies and/or apheresis, to achieve goals of low-density lipoprotein cholesterol (LDL-C) reduction by 50% or to 100 mg/dL. METHODS: In this study, Japanese patients with HoFH on stable LLT and diet were treated with Lomitapide, initiated at 5 mg/day and escalated to maximum tolerated dose (up to 60 mg/day) over 14 weeks. The primary efficacy endpoint was mean percentage change from baseline to Week 26 in LDL-C. Secondary endpoints included changes in other lipid parameters and safety throughout the 56-week study (including follow-up). RESULTS: Nine patients entered the efficacy phase of the study and, of these, eight completed 56 weeks. Mean LDL-C was reduced by 42% (p0.0001) at 26 weeks, from 199 mg/dL (95% CI: 149-250) at baseline to 118 mg/dL (95% CI: 70-166). A 50% reduction in LDL-C and LDL-C 100 mg/dL was achieved by five and six of nine patients, respectively, at 26 weeks. After 56 weeks, LDL-C was reduced by 38% (p=0.0032) from baseline. Significant reductions in non-HDL-C, VLDL-C, triglycerides, and apolipoprotein B were also reported at Week 26. There were no new safety signals and, similar to previous studies, gastrointestinal adverse events were the most common adverse events. CONCLUSION: Lomitapide, added to ongoing treatment with other LLTs, was effective in rapidly and significantly reducing the levels of LDL-C and other atherogenic apolipoprotein B-containing lipoproteins in adult Japanese patients with HoFH.


