Timolol MaleateCAS# 26921-17-5 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26921-17-5 | SDF | Download SDF |
| PubChem ID | 5281056 | Appearance | Powder |
| Formula | C17H28N4O7S | M.Wt | 432.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (231.22 mM; Need ultrasonic) | ||
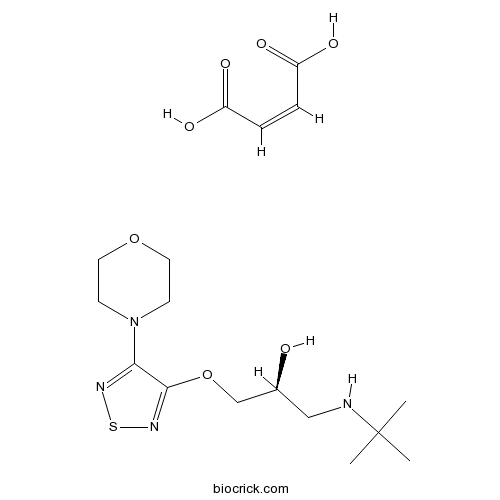
| Chemical Name | (Z)-but-2-enedioic acid;(2S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol | ||
| SMILES | CC(C)(C)NCC(COC1=NSN=C1N2CCOCC2)O.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | WLRMANUAADYWEA-NWASOUNVSA-N | ||
| Standard InChI | InChI=1S/C13H24N4O3S.C4H4O4/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17;5-3(6)1-2-4(7)8/h10,14,18H,4-9H2,1-3H3;1-2H,(H,5,6)(H,7,8)/b;2-1-/t10-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | β1-adrenergic blocker. |

Timolol Maleate Dilution Calculator

Timolol Maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3122 mL | 11.561 mL | 23.1219 mL | 46.2438 mL | 57.8048 mL |
| 5 mM | 0.4624 mL | 2.3122 mL | 4.6244 mL | 9.2488 mL | 11.561 mL |
| 10 mM | 0.2312 mL | 1.1561 mL | 2.3122 mL | 4.6244 mL | 5.7805 mL |
| 50 mM | 0.0462 mL | 0.2312 mL | 0.4624 mL | 0.9249 mL | 1.1561 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2312 mL | 0.4624 mL | 0.578 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(S)-Timolol maleate, is a potent non-selective β-adrenergic receptor antagonist (Ki values are 1.97 and 2.0 nM for β1 and β2 receptor subtypes respectively).
- H-D-Dab-OH.2HCl
Catalog No.:BCC3185
CAS No.:26908-94-1
- Fmoc-Orn(Dde)-OH
Catalog No.:BCC3534
CAS No.:269062-80-8
- Etravirine (TMC125)
Catalog No.:BCC5027
CAS No.:269055-15-4
- 2(-4-Chloro-3-hydroxy-1-butynyl)-5-1,(3-pentadiynyl)thiophene
Catalog No.:BCN1465
CAS No.:26905-70-4
- Oxysophocarpine
Catalog No.:BCN5156
CAS No.:26904-64-3
- 6-Hydroxybenzofuran-2(3H)-one
Catalog No.:BCN5155
CAS No.:2688-49-5
- Isomahanimbine
Catalog No.:BCN3175
CAS No.:26871-46-5
- 1,3-Bis[2-(4-aminophenyl)-2-propyl]benzene
Catalog No.:BCC8419
CAS No.:2687-27-6
- Penfluridol
Catalog No.:BCC4696
CAS No.:26864-56-2
- Triptohypol F
Catalog No.:BCN5154
CAS No.:268541-26-0
- Homoharringtonine
Catalog No.:BCN4958
CAS No.:26833-87-4
- Harringtonine
Catalog No.:BCN6794
CAS No.:26833-85-2
- Aglaxiflorin D
Catalog No.:BCN6594
CAS No.:269739-78-8
- 16-Acetoxy-7-O-acetylhorminone
Catalog No.:BCN5157
CAS No.:269742-39-4
- Sphingosine-1-phosphate
Catalog No.:BCC7034
CAS No.:26993-30-6
- Lariciresinol
Catalog No.:BCN5158
CAS No.:27003-73-2
- 3,4-Dichloro-Phe-OMe.HCl
Catalog No.:BCC2635
CAS No.:270063-47-3
- 17-Hydroxy-1a,2a-methylenepregna-4,6-diene-3,20-dione acetate
Catalog No.:BCC8442
CAS No.:2701-50-0
- alpha-Hederin
Catalog No.:BCN5159
CAS No.:27013-91-8
- 2'-Methoxykurarinone
Catalog No.:BCN2986
CAS No.:270249-38-2
- H-D-Glu(OMe)-OMe.HCl
Catalog No.:BCC2941
CAS No.:27025-25-8
- Solasurine
Catalog No.:BCN2694
CAS No.:27028-76-8
- Paradol
Catalog No.:BCC1837
CAS No.:27113-22-0
- MMK 1
Catalog No.:BCC6037
CAS No.:271246-66-3
Sustained Delivery of Timolol Maleate for Over 90 Days by Subconjunctival Injection.[Pubmed:27835065]
J Ocul Pharmacol Ther. 2016 Dec;32(10):642-649.
PURPOSE: Medical treatment of glaucoma relies on intraocular pressure (IOP)-lowering medications, typically administered daily by the patient. While these medications are effective when applied correctly, patient adherence is a major obstacle in glaucoma treatment. We have developed a sustained-release formulation of Timolol Maleate that can be injected subconjunctivally to avoid patient noncompliance. METHODS: A biodegradable microsphere formulation for Timolol Maleate was injected subconjunctivally in normal rabbits. We measured timolol levels in tears, aqueous humor, vitreous humor, and serum of study rabbits. Furthermore, IOP profiles were recorded longitudinally. Tissue compatibility and side effects were evaluated using histochemistry. RESULTS: The microsphere formulation led to measureable amounts of timolol in the aqueous humor and the tear film for up to 90 days. Timolol was not detectable in the serum at any time. A significant reduction of IOP was observed in treated eyes. Clinically, the subconjunctival administration of the microspheres was well tolerated with no signs of inflammation or infection. The absence of local inflammation was confirmed by histology. CONCLUSIONS: A single subconjunctival administration of timolol microspheres achieved delivery and IOP reduction in rabbits for up to 90 days without local or systemic inflammation or toxicity. This approach has the potential to improve the management of glaucoma in patient populations, who are challenged to adhere to a regimen of daily eye drops.
Fixed combination of travoprost and timolol maleate reduces intraocular pressure in Japanese patients with primary open-angle glaucoma or ocular hypertension: analysis by prostaglandin analogue.[Pubmed:28053501]
Clin Ophthalmol. 2016 Dec 20;11:55-61.
BACKGROUND: We have shown a decrease in mean intraocular pressure (IOP) by switching to travoprost/timolol fixed combination (TTFC) in subjects receiving prostaglandin analogue (PGA) monotherapy and requiring additional medication in a previous report. For analyzing factors affecting IOP reduction, baseline IOP and preceding PGA were selected as statistically and clinically significant factors. In this report, we examine IOP-lowering effect and adverse drug reactions by preceding PGA. METHODS: Patients with primary open angle glaucoma or ocular hypertension who received monotherapy with one of four PGAs (travoprost, latanoprost, tafluprost, or bimatoprost) for at least 3 months at 26 institutions and were determined to require additional medication by their primary physician were included. IOP reduction and adverse events were examined at 4, 8, and 12 weeks for each of four PGAs after switching to TTFC. RESULTS: In total, 157 patients who could be followed up for at least 4 weeks after switching to TTFC were included in the efficacy analysis. Multiple regression analysis was performed, and baseline IOP and PGA were found to be significant factors to IOP reduction. IOP reduction at week 12, adjusted with the regression model, was -3.5, -1.8, and -1.4 mmHg in the tafluprost, latanoprost, and travoprost groups, whereas it was -0.5 mmHg in the bimatoprost group. Along with differences in baseline IOP between groups, an IOP-lowering effect of >1 mmHg was noted in the tafluprost, latanoprost, and travoprost groups after the switch. IOP was maintained at 13.8-14.8 mmHg throughout the follow-up period. No serious adverse events or noteworthy issues were observed in any group after the switch. CONCLUSION: Clinically significant IOP-reducing effects of TTFC were observed in the latanoprost, travoprost, and tafluprost groups when switching from each PGA monotherapy, while there were some differences in effects between groups, with minimal safety concerns.
A New UPLC Method with Chemometric Design-Optimization Approach for the Simultaneous Quantitation of Brimonidine Tartrate and Timolol Maleate in an Eye Drop Preparation.[Pubmed:27881494]
J Chromatogr Sci. 2017 Feb;55(2):154-161.
A new ultra-performance liquid chromatography (UPLC) with photodiode array was proposed for the quantitation of Brimonidine Tartrate (BRI) and Timolol Maleate (TIM) in eye drop using experimental design and optimization methodology. A 3(3) full factorial design was applied to uncover the effects of the selected factors and their interactions on the chromatographic response function for the optimization of experimental conditions in the development of a new UPLC method. As a result, the optimal chromatographic conditions giving a better separation and short analysis time were found to be 49.2 degrees C for column temperature; 0.38 mL/min for flow rate and 56.7 % (v/v) for 0.1 M CH3COOH used in mobile phase. The elution of BRI and TIM was reported as 0.508 and 0.652 min within a short runtime of 1.5 min, respectively. Calibration graphs for BRI and TIM were obtained by the regression of the concentration on the peak area, which was detected at 246 and 298 nm, respectively. The method validation was performed by the analysis of the synthetic mixtures, intra-day and inter-day samples and standard addition samples. This study shows that the optimized and validated UPLC method is very promising and available for the quantification of BRI and TIM in an eye drop formulation.
[Chinese expert consensus on the use of topical timolol maleate treatment of infantile hemangiomas].[Pubmed:28275803]
Shanghai Kou Qiang Yi Xue. 2016 Dec;25(6):744-747.
Non-selective beta-blocker propranolol has been proved by FDA as the first-line agent for infantile hemangioma (IH) with dramatic response. To reduce the side effects caused by systemic administration of propranolol, Timolol Maleate treatment has been increasingly used as an alternative to systemic beta-blockers and watchful waiting for many IH patients in recent years. However, the appropriate indications, drug dosage, dosing regimen, time for initiation, optimal duration, monitoring for side effects still remains controversial. To standardize the use of topical timolol in treating IH, avoid overtreatment or under-treatment, as well as minimize complications, a Chinese expert consensus on the use of topical timolol treatment of IH has been approved and written by a multidisciplinary experts group based on an up-to-date literature review and repeated discussion, which can be used to reduce inappropriate variations in clinical practice and to promote the delivery of high quality, evidence-based health care for IH patients.
Beta-adrenoceptor antagonist activities and binding affinities of timolol enantiomers in rat atria.[Pubmed:2573714]
J Pharm Pharmacol. 1989 Sep;41(9):649-50.
S-Timolol is an effective anti-glaucoma drug, but has potentially hazardous side effects. Recently, R-timolol, also, has been reported to be effective in lowering elevated intraocular pressure. In the present study, the beta-adrenoceptor antagonist activities and binding of R- and S-enantiomers of timolol have been examined on rat atrial preparations. The beta-antagonistic activities were investigated using spontaneously beating rat heart atria. Both timolol enantiomers inhibited (-)-isoprenaline-induced chronotropic action competitively. S-Timolol was about 54 times more potent than R-timolol. The apparent binding affinities of timolol enantiomers to beta 1- and beta 2-adrenoceptors were determined by a radioligand binding assay using (-)-[125I]iodocyanopindolol (ICYP) as a marker and CGP 20712 A as a beta 1- and ICI 118,551 as a beta 2-adrenoceptor antagonist. Both enantiomers of timolol inhibited ICYP binding in nanomolar concentrations with Hill coefficients near unity. Neither enantiomer showed selectivity between beta 1- and beta 2-adrenoceptors, but R-timolol was approximately 30 times less active than S-timolol. It is concluded that R-timolol is a relatively potent non-selective beta-adrenoceptor blocking agent, but may possibly exert a more localized beta-adrenoceptor action in the eye than S-timolol, thus improving the safety of ocular timolol therapy.


