T0901317Liver X receptor agonist,potent and selective CAS# 293754-55-9 |
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- LXR-623
Catalog No.:BCC4273
CAS No.:875787-07-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 293754-55-9 | SDF | Download SDF |
| PubChem ID | 447912 | Appearance | Powder |
| Formula | C17H12F9NO3S | M.Wt | 481.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (207.76 mM) Methanol : ≥ 100 mg/mL (207.76 mM) *"≥" means soluble, but saturation unknown. | ||
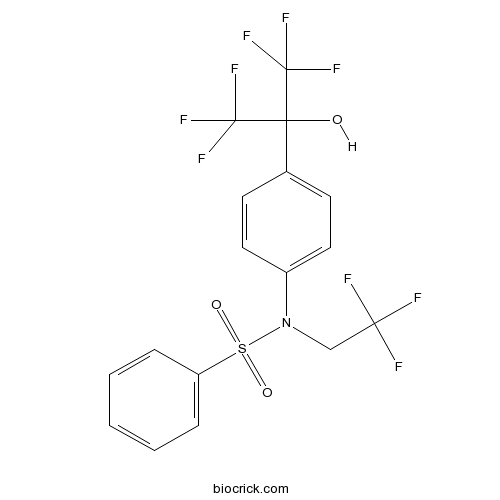
| Chemical Name | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide | ||
| SMILES | C1=CC=C(C=C1)S(=O)(=O)N(CC(F)(F)F)C2=CC=C(C=C2)C(C(F)(F)F)(C(F)(F)F)O | ||
| Standard InChIKey | SGIWFELWJPNFDH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, high affinity liver X receptor (LXR) agonist (EC50 ~ 50 nM, Kd values are 7 and 22 nM for LXR-α and LXR-β respectively). Upregulates expression of the ABCA1 gene associated with cholesterol efflux regulation and HDL metabolism. Decreases amyloid-β production in primary neurons in vitro. Displays an EC50 of ~ 5 μM for activation of bile acid farnesoid X receptors (FXRs); 10-fold more potent than natural FXR ligand chenodeoxycholic acid. Also exhibits inverse agonist activity at constitutive androstane receptors (CAR). |

T0901317 Dilution Calculator

T0901317 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0776 mL | 10.3879 mL | 20.7758 mL | 41.5515 mL | 51.9394 mL |
| 5 mM | 0.4155 mL | 2.0776 mL | 4.1552 mL | 8.3103 mL | 10.3879 mL |
| 10 mM | 0.2078 mL | 1.0388 mL | 2.0776 mL | 4.1552 mL | 5.1939 mL |
| 50 mM | 0.0416 mL | 0.2078 mL | 0.4155 mL | 0.831 mL | 1.0388 mL |
| 100 mM | 0.0208 mL | 0.1039 mL | 0.2078 mL | 0.4155 mL | 0.5194 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
T0901317 is an agonist for multiple targets, which possesses EC50 values of 20 nM and 5 μM for LXRα and FXR, respectively. Furthermore, it is RORα and RORγ dual inverse agonist with estimated IC50 of 2.0 μM and 1.7 μM, respectively. [1, 2]
LXRs play central roles in cholesterol metabolism, which are nuclear receptors that regulate the metabolism of several important lipids, including cholesterol and bile acids. LXRs were first identified as orphan members of the nuclear receptor superfamily. Like LXRs, FXR was also initially thought as an orphan member of the nuclear receptor superfamily. But FXR was later identified as the physiological receptor for bile acids and shown to regulate a feedback loop for bile acid transport and synthesis as well as modulating additional functions in lipid metabolism. Retinoic acid receptor-related orphan receptors (RORs) are important in a variety of physiological processes including hepatic gluconeogenesis, lipid metabolism, circadian rhythm, and immunological functions. T0901317 inhibited transactivation activity of RORα and RORγ by direct binding with high affinity which led to the regulation of the receptor’s ability to interact with transcriptional cofactor proteins, but did not show inhibitory activity against RORβ. [1, 2]
A short synthetic rhodamine-labeled peptide was used to conduct a fluorescence polarization assay for T0901317 binding to LXRα. In this homogeneous biochemical assay, the greater the extent of rhodamine-peptide binding to LXR, the greater the extent of fluorescence polarization observed. The potency of T0901317 binding to LXRα was determined, which possessed EC50 of 20 nM. Adopting HEK293 cells transfected with Gal4 DBD-FXR ligand-binding-domain chimeric receptor along with Gal4-responsive luciferase reporter, T0901317 activated FXR with an EC50 of ~5 uM, which surpassed the potency of natural FXR ligand. Moreover, using a cell-based GAL4-NR LBD cotransfection assay, it was found that T0901317 at a dose of 2 μM was a potent in repressing both GAL4-RORα and GAL4-RORγ. Furthermore, T0901317 was demonstrated its selectivity that T0901317 inhibited the constitutive transactivation activity of both GAL4-RORα and GAL4-RORγ with little or no activity on GAL4-RORβ T0901317 demonstrated an excellent dose response, with an estimated IC50 values of 2.0 for RORα and 1.7 μM for RORγ, respectively. [1, 2, 3]
The role of T0901317 on ABCA1 expression in vivo was also studied. We treated 11-week-old APP23 mice orally by gastric gavage for 6 days with 50 mg/kg/day T0901317. The treatment of APP23 mice resulted in a substantial increase in the expression of ABCA1, however the expression of APP did not change. [4]
References:
[1]. Schultz J R, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis[J]. Genes & development, 2000, 14(22): 2831-2838.
[2]. Kumar N, Solt L A, Conkright J J, et al. The benzenesulfoamide T0901317 [N-(2, 2, 2-trifluoroethyl)-N-[4-[2, 2, 2-trifluoro-1-hydroxy-1-(trifluoromethyl) ethyl] phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-α/γ inverse agonist[J]. Molecular pharmacology, 2010, 77(2): 228-236.
[3]. Houck K A, Borchert K M, Hepler C D, et al. T0901317 is a dual LXR/FXR agonist[J]. Molecular genetics and metabolism, 2004, 83(1): 184-187.
[4]. Liu Y, Yan C, Wang Y, et al. Liver X receptor agonist T0901317 inhibition of glucocorticoid receptor expression in hepatocytes may contribute to the amelioration of diabetic syndrome in db/db mice[J]. Endocrinology, 2006, 147(11): 5061-5068.
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
Functional induction of P-glycoprotein efflux pump by phenyl benzenesulfonamides: Synthesis and biological evaluation of T0901317 analogs.[Pubmed:27497733]
Eur J Med Chem. 2016 Oct 21;122:744-755.
N-(2,2,2-Trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl ]phenyl]-benzenesulfonamide (T0901317, 6) is a potent activator of pregnane-X-receptor (PXR), which is a nuclear receptor controlling P-gp expression. Herein, we aimed to investigate P-gp induction activity of T0901317 and establish its structure-activity relationship. T0901317 along with a series of N-triazolyl-methylene-linked benzenesulfonamides were synthesized and screened for P-gp induction activity using a rhodamine-123 based efflux assay in the P-gp overexpressing human adenocarcinoma LS-180 cells, wherein several compounds showed potent P-gp induction activity at 5 muM. Treatment with benzene sulphonamides led to the decrease in intracellular accumulation of a fluorescent P-gp substrate rhodamine-123 up to 48% (control 100%). In the western-blot studies, T0901317 (6) and its triazole linked analog 26e at 5 muM displayed induction of P-gp expression in LS180 cells. These compounds were non-toxic in LS-180 and human neuroblastoma SH-SY5Y cells (IC50 > 50 muM). The compound 26e showed significant P-gp induction even at 0.3 muM, indicating an excellent therapeutic window. These results clearly indicate promise of this class of compounds as potential agents to enhance amyloid-beta clearance in Alzheimers patients.
LXR agonist T0901317 upregulates thrombomodulin expression in glomerular endothelial cells by inhibition of nuclear factorkappaB.[Pubmed:27082844]
Mol Med Rep. 2016 Jun;13(6):4888-96.
Dysfunction of glomerular endothelial cells (GECs) induces a variety of symptoms, including proteinuria, inflammation, vascular diseases, fibrosis and thrombosis. Thrombomodulin (TM) acts as a vasoprotective molecule on the surface of the vascular endothelial cells to maintain the homeostasis of the endothelial microenvironment by suppressing cellular proliferation, adhesion and inflammatory responses. Liver X receptor (LXR), a nuclear receptor (NR) and a bile acidactivated transcription factor, regulates metabolism and cholesterol transport, vascular tension and inflammation. Previous studies indicated that TM expression is upregulated by various NRs; however, it is unclear whether pharmacological modulation of LXR may affect TM expression and GEC function. The current study revealed that LXR activation by its agonist, T0901317, upregulates the expression and activity of TM. This effect was mediated specifically through LXRalpha, and not through LXRbeta. Additionally, T0901317 treatment inhibited nuclear factorkappaB (NFkappaB) signaling and the secretion of high glucoseinduced proinflammatory mediators, including tumor necrosis factoralpha and interleukin1beta in GECs. Coimmunoprecipitation experiments determined that treatment with T0901317 enhances the interaction between LXRalpha and the transcriptional coactivator, p300, in GEC extracts. The present findings suggest that NFkappaB may be a negative regulator of TM expression, and its removal may contribute to TM gene expression, particularly when in competition with the T0901317enhanced formation of the LXR/p300 complex. Therefore, LXR may be a novel molecular target for manipulating TM in GECs, which may advance the treatment of endothelial cellassociated diseases.
Liver X receptor agonist T0901317 reverses resistance of A549 human lung cancer cells to EGFR-TKI treatment.[Pubmed:28097086]
FEBS Open Bio. 2016 Dec 20;7(1):35-43.
Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) is effective in lung cancer patients carrying sensitive EGFR mutations. In this study, we investigated if liver X receptor (LXR) agonist T0901317 could reverse the resistance of lung cancer cell lines A549 and H1650 to EGFR-TKI treatment. We found that T0901317 could make natural EGFR-TKI-resistant A549 human lung cancer cells sensitive to EGFR-TKI treatment and that this was dependent on LXRbeta expression. However, T0901317 does not have a similar effect on another natural EGFR-TKI-resistant cell line H1650.
Altered activity profile of a tertiary silanol analog of multi-targeting nuclear receptor modulator T0901317.[Pubmed:26905831]
Bioorg Med Chem Lett. 2016 Apr 1;26(7):1817-20.
We report the design, synthesis, and physicochemical/biological evaluation of novel silanol derivative 6 (sila-T) as a silanol analog of multi-target nuclear receptor modulator T0901317 (5). Compound 6 showed intermediate hydrophobicity between the corresponding alcohol 13 and perfluoroalcohol 5. While 5 exhibited potent activities toward liver X receptor alpha and beta, farnesoid X receptor, pregnane X receptor (PXR) and retinoic acid receptor-related orphan receptor (ROR)gamma, silanol 6 exhibited activity only toward PXR and RORs. Incorporation of silanol instead of perfluoroalcohol is a promising option for developing novel target-selective, biologically active compounds.
TO901317, a potent LXR agonist, is an inverse agonist of CAR.[Pubmed:23665929]
J Toxicol Sci. 2013;38(3):309-15.
The basal transcriptional activity of unliganded human constitutive androstane receptor (hCAR) was shown to be repressed by the potent liver X receptor (LXR) agonist, TO901317, in a concentration-dependent manner using a reporter assay in cultured cells. TO901317 also repressed the basal transcriptional activity of both mouse and rat CAR. The certified hCAR agonist, CITCO, partially reversed this repressive effect of TO901317 on hCAR basal activity. Unlike hCAR, a three alanine insertion mutant and the splice variant 2 of hCAR require agonists, such as CITCO, to become transcriptionally active and the CITCO-induced reporter activity was repressed by TO901317. As has been previously shown for the typical hCAR inverse agonist, PK11195, TO901317 blocked the interaction of hCAR with steroid receptor co-activator 1 (SRC1). In contrast, the interaction between hCAR and nuclear receptor corepressor 1 (NCoR1) was promoted by PK11195 and TO901317. Furthermore, the hCAR-mediated basal induction of endogenous cytochrome P450 2B6 (CYP2B6) mRNA was adversely affected by co-treatment with TO901317.
The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease.[Pubmed:15557325]
J Biol Chem. 2005 Feb 11;280(6):4079-88.
Recent studies indicate that oxysterols, which are ligands for the nuclear hormone liver X receptors (LXR), decrease amyloid beta (Abeta) secretion in vitro. The effect was attributed primarily to the ATP-binding cassette transporter A1 (ABCA1) transcriptionally up-regulated by ligand-activated LXRs. We now examined the effect of the synthetic LXR ligand T0901317, which can be used in vivo, on Abeta production in vitro and in APP23 transgenic mice. T0901317 applied to a variety of in vitro models, including immortalized fibroblasts from Tangier patients, and primary embryonic mouse neurons caused a concentration-dependent decrease in Abeta secretion, and this effect was increased by the addition of apolipoprotein A-I. The inhibition of Abeta production by T0901317 was cell-type specific, being more prominent in primary neurons than in non-neuronal cells. Tangier fibroblasts lacking a functional ABCA1 secreted more Abeta than control fibroblasts, thus demonstrating the role of ABCA1 in amyloid precursor protein (APP) processing and Abeta generation. T0901317 treatment of 11-week-old APP23 mice for 6 days showed a significant increase in ABCA1 expression and a decrease in the ratio of soluble APP (sAPP)beta- to sAPPalpha-cleavage products. Most importantly, the treatment caused a statistically significant reduction in the levels of soluble Abeta40 and of Abeta42 in the brain these mice. Our experiments demonstrate that T0901317 decreases amyloidogenic processing of APP in vitro and in vivo, thus supporting the search for potent and specific LXR ligands with properties allowing therapeutic application.
Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers.[Pubmed:10968783]
Science. 2000 Sep 1;289(5484):1524-9.
Several nuclear hormone receptors involved in lipid metabolism form obligate heterodimers with retinoid X receptors (RXRs) and are activated by RXR agonists such as rexinoids. Animals treated with rexinoids exhibited marked changes in cholesterol balance, including inhibition of cholesterol absorption and repressed bile acid synthesis. Studies with receptor-selective agonists revealed that oxysterol receptors (LXRs) and the bile acid receptor (FXR) are the RXR heterodimeric partners that mediate these effects by regulating expression of the reverse cholesterol transporter, ABC1, and the rate-limiting enzyme of bile acid synthesis, CYP7A1, respectively. Thus, these RXR heterodimers serve as key regulators of cholesterol homeostasis by governing reverse cholesterol transport from peripheral tissues, bile acid synthesis in liver, and cholesterol absorption in intestine.


