CyclenCAS# 294-90-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 294-90-6 | SDF | Download SDF |
| PubChem ID | 64963 | Appearance | White crystal |
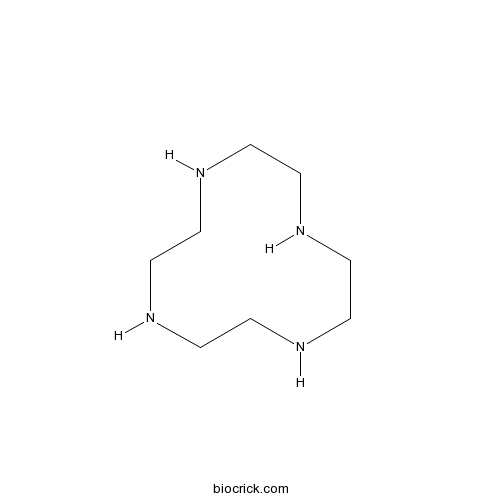
| Formula | C8H20N4 | M.Wt | 172.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,4,7,10-tetrazacyclododecane | ||
| SMILES | C1CNCCNCCNCCN1 | ||
| Standard InChIKey | QBPPRVHXOZRESW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H20N4/c1-2-10-5-6-12-8-7-11-4-3-9-1/h9-12H,1-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cyclen Dilution Calculator

Cyclen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8048 mL | 29.0242 mL | 58.0484 mL | 116.0968 mL | 145.121 mL |
| 5 mM | 1.161 mL | 5.8048 mL | 11.6097 mL | 23.2194 mL | 29.0242 mL |
| 10 mM | 0.5805 mL | 2.9024 mL | 5.8048 mL | 11.6097 mL | 14.5121 mL |
| 50 mM | 0.1161 mL | 0.5805 mL | 1.161 mL | 2.3219 mL | 2.9024 mL |
| 100 mM | 0.058 mL | 0.2902 mL | 0.5805 mL | 1.161 mL | 1.4512 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- Cypellocarpin C
Catalog No.:BCN7556
CAS No.:294856-66-9
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
Synthesis of Symmetrical Tetrameric Conjugates of the Radiolanthanide Chelator DOTPI for Application in Endoradiotherapy by Means of Click Chemistry.[Pubmed:29692987]
Front Chem. 2018 Apr 10;6:107.
Due to its 4 carbonic acid groups being available for bioconjugation, the Cyclen tetraphosphinate chelator DOTPI, 1,4,7,10-tetraazacyclododecane-1,4,7, 10-tetrakis[methylene(2-carboxyethylphosphinic acid)], represents an ideal scaffold for synthesis of tetrameric bioconjugates for labeling with radiolanthanides, to be applied as endoradiotherapeuticals. We optimized a protocol for bio-orthogonal DOTPI conjugation via Cu(I)-catalyzed Huisgen-cycloaddition of terminal azides and alkynes (CuAAC), based on the building block DOTPI(azide)4. A detailed investigation of kinetic properties of Cu(II)-DOTPI complexes aimed at optimization of removal of DOTPI-bound copper by transchelation. Protonation and equilibrium properties of Ca(II)-, Zn(II), and Cu(II)-complexes of DOTPI and its tetra-cyclohexylamide DOTPI(Chx)4 (a model for DOTPI conjugates) as well as kinetic inertness (transchelation challenge in the presence of 20 to 40-fold excess of EDTA) were investigated by pH-potentiometry and spectrophotometry. Similar stability constants of Ca(II)-, Zn(II), and Cu(II)-complexes of DOTPI (logK(CaL) = 8.65, logK(ZnL = 15.40, logK(CuL) = 20.30) and DOTPI(Chx)4 (logK(CaL) = 8.99, logK(ZnL) = 15.13, logK(CuL) = 20.42) were found. Transchelation of Cu(II)-complexes occurs via proton-assisted dissociation, whereafter released Cu(II) is scavenged by EDTA. The corresponding dissociation rates [kd = 25 x 10(-7) and 5 x 10(-7) s(-1) for Cu(DOTPI) and Cu(DOTPI(Chx)4), respectively, at pH 4 and 298 K] indicate that conjugation increases the kinetic inertness by a factor of 5. However, demetallation is completed within 4.5 and 7.2 h at pH 2 and 25 degrees C, respectively, indicating that Cu(II) removal after formation of CuAAC can be achieved in an uncomplicated manner by addition of excess H4EDTA. For proof-of-principle, tetrameric DOTPI conjugates of the prostate-specific membrane antigen (PSMA) targeting motif Lys-urea-Glu (KuE) were synthesized via CuAAC as well as dibenzo-azacyclooctine (DBCO) based, strain-promoted click chemistry (SPAAC), which were labeled with Lu-177 and subsequently evaluated in vitro and in SCID mice bearing subcutaneous LNCaP tumor (PSMA+ human prostate carcinoma) xenografts. High affinities (3.4 and 1.4 nM, respectively) and persistent tumor uptakes (approx. 3.5% 24 h after injection) confirm suitability of DOTPI-based tetramers for application in targeted radionuclide therapy.
Small Gd(III) Tags for Gd(III)-Gd(III) Distance Measurements in Proteins by EPR Spectroscopy.[Pubmed:29629761]
Inorg Chem. 2018 May 7;57(9):5048-5059.
The C7-Gd and C8-Gd tags are compact hydrophilic Cyclen-based lanthanide tags for conjugation to cysteine residues in proteins. The tags are enantiomers, which differ in the configuration of the 2-hydroxylpropyl pendant arms coordinating the lanthanide ion. Here, we report the electron paramagnetic resonance (EPR) performance of the C7-Gd ( S configuration) and C8-Gd ( R configuration) tags loaded with Gd(III) on two mutants of the homodimeric ERp29 protein. The W-band EPR spectra were found to differ between the tags in the free state and after conjugation to the protein. In addition, the spectra were sensitive to the labeling position, which may originate from an environment-dependent charge density on the Gd(III)-coordinating oxygens. This is in agreement with previous NMR experiments with different lanthanide ions, which suggested sensitivity to H-bonding. W-band (1)H-ENDOR (electron-electron double resonance) experiments detected effects from orientation selection in the central transition, due to a relatively narrow distribution in the ZFS parameters as indicated by simulations. In contrast, the distance distributions derived from DEER (double electron-electron resonance) measurements were insensitive to the R or S configuration of the tags and did not exhibit any orientation selection effects. The DEER measurements faithfully reflected the different widths of the distance distributions at the different protein sites in agreement with previous DEER measurements using other Gd(III) tags. Due to their small size, short tether to the protein, and a broad central EPR transition, the C7-Gd and C8-Gd tags are attractive Gd(III) tags for measurements of relatively short (<4 nm) distances by EPR spectroscopy.
Multiply Intercalator-Substituted Cu(II) Cyclen Complexes as DNA Condensers and DNA/RNA Synthesis Inhibitors.[Pubmed:29683319]
Inorg Chem. 2018 May 7;57(9):5004-5012.
Many drugs that are applied in anticancer therapy such as the anthracycline doxorubicin contain DNA-intercalating 9,10-anthraquinone (AQ) moieties. When Cu(II) Cyclen complexes were functionalized with up to three (2-anthraquinonyl)methyl substituents, they efficiently inhibited DNA and RNA synthesis resulting in high cytotoxicity (selective for cancer cells) accompanied by DNA condensation/aggregation phenomena. Molecular modeling suggests an unusual bisintercalation mode with only one base pair between the two AQ moieties and the metal complex as a linker. A regioisomer, in which the AQ moieties point in directions unfavorable for such an interaction, had a much weaker biological activity. The ligands alone and corresponding Zn(II) complexes (used as redox inert control compounds) also exhibited lower activity.
Intra-cavity proton bonding and anharmonicity in the anionophore cyclen.[Pubmed:29557457]
Phys Chem Chem Phys. 2018 Mar 28;20(13):8968-8975.
Proton bonding drives the supramolecular chemistry of a broad range of materials with polar moieties. Proton delocalization and electronic charge redistribution have a profound impact on the structure of proton-bound molecular frameworks, and pose fundamental challenges to quantum chemical modelling. This study provides insights into the structural and spectral signatures of the intramolecular proton bond formed in a benchmark polyazamacrocycle anionophore (Cyclen, 1,4,7,10-tetraazacyclododecane). Infrared action spectroscopy is employed to characterize the macrocycle, isolated in protonated form. In its most stable configuration, protonated Cyclen adopts an open arrangement of Cs symmetry with a particularly strong NHdelta+N bond across the cavity. The quantum chemical analysis of the infrared spectrum reveals intrinsic difficulties for the accurate description of the vibrational modes of the system. The reconciliation of the computational predictions with experiment demands a careful anharmonic treatment of the proton motion, which exposes the limitations of current methods. Best results are obtained with the incorporation of anharmonicity only to the fundamental modes directly related to motions of the proton. However, the full anharmonic treatment of the system fails to describe correctly the vibrations related to the macrocycle backbone. The results should serve as motivation for new developments in the modelling of proton bonded systems.
Luminescent europium sensors for specific detection of 8-oxo-dGTP by time-gated fluorescence.[Pubmed:29731311]
Bioorg Med Chem. 2018 Jul 23;26(12):3254-3260.
The 9-hydroxy-1,3-diazaphenoxazine-2-one unit was conjugated with the Eu(3+)-Cyclen complex through a linker. This diazaphenoxazine group was expected as an antenna unit for the excitation of europium ion, and a selective recognition site for 8-oxo-dGTP base. Among the synthesized three derivatives, the highest fluorescence emission was obtained by the complex constructed of an ethylene linker and the Cyclen unit with three N,N-dimethylacetamide groups. The Eu(3+)-Cyclen complex exhibited a selective response to the 8-oxo-dGTP in aqueous media by a time-resolved fluorescence assay.
When Is Ligand p Ka a Good Descriptor for Catalyst Energetics? In Search of Optimal CO2 Hydration Catalysts.[Pubmed:29665337]
J Phys Chem A. 2018 May 10;122(18):4579-4590.
We present a detailed study of nearly 70 Zn molecular catalysts for CO2 hydration from four diverse ligand classes ranging from well-studied carbonic anhydrase mimics (e.g., Cyclen) to new structures we obtain by leveraging diverse hits from large organic libraries. Using microkinetic analysis and establishing linear free energy relationships, we confirm that turnover is sensitive to the relative thermodynamic stability of reactive hydroxyl and bound bicarbonate moieties. We observe a wide range of thermodynamic stabilities for these intermediates, showing up to 6 kcal/mol improvement over well-studied Cyclen catalysts. We observe a good correlation between the p Ka of the Zn-OH2 moiety and the resulting relative stability of hydroxyl moieties over bicarbonate, which may be rationalized by the dominant effect of the difference in higher Zn-OH bond order in comparison to weaker bonding in bicarbonate and water. A direct relationship is identified between isolated organic ligand p Ka and the p Ka of a bound water molecule on the catalyst. Thus, organic ligand p Ka, which is intuitive, easy to compute or tabulate, and much less sensitive to electronic structure method choice than whole-catalyst properties, is a good quantitative descriptor for predicting the effect of through-bond electronic effects on relative CO2 hydration energetics. We expect this to be applicable to other reactions where is it essential to stabilize turnover-determining hydroxyl species with respect to more weakly bound moieties. Finally, we note exceptions for rigid ligands (e.g., porphyrins) that are observed to preferentially stabilize hydroxyl over bicarbonate without reducing p Ka values as substantially. We expect the strategy outlined here, to (i) curate diverse ligands from large organic libraries and (ii) identify when ligand-only properties can determine catalyst energetics, to be broadly useful for both experimental and computational catalyst design.
The Impact of Hormonal Contraceptives on Breast Cancer Pathology.[Pubmed:29687205]
Horm Cancer. 2018 Aug;9(4):240-253.
This retrospective case series study, using data obtained through questionnaires and histopathological diagnoses from 656 patients enrolled in the Department of Defense (DoD) Clinical Breast Care Project (CBCP), evaluated associations between hormonal contraceptive use and breast cancer pathology including benign breast pathologies. Three combination hormonal contraceptive agents (COCs) Lo Ovral (LO), Ortho Novum (ON), and Ortho Tri-Cyclen (OTC) were evaluated as they represented the most commonly used hormonal contraceptives in our cohort. The results of this study suggest that the ever use of LO + ON + OTC does not influence the overall incidence of benign breast condition or malignant disease compared to other COCs; however, patients that have used OTC had an association with a diagnosis of benign or luminal A pathologies whereas ON was associated with a diagnosis of benign and DCIS; LO showed no association with any diagnosis-benign or malignant. Patients that have used LO or ON were more likely to be diagnosed with breast cancer at age >/= 40 years whereas patients that had ever used OTC were likely to be diagnosed before the age of 40. Caucasians were less likely to have used OTC and more likely to have used ON; however, use of either hormonal agent positively correlated with premenopausal status at diagnosis and having a benign condition. Age at diagnosis, ethnicity, BMI, family history, menstruation status, and duration of use were all independent predictors of different histopathological subtypes. We conclude that patient-specific variables should be considered when deciding on which type of hormonal contraceptive to use to minimize the risk of developing breast cancer or a breast-related pathology.
Steric Effects on the Binding of Phosphate and Polyphosphate Anions by Zinc(II) and Copper(II) Dinuclear Complexes of m-Xylyl-bis-cyclen.[Pubmed:29749744]
Inorg Chem. 2018 Jun 4;57(11):6466-6478.
The triethylbenzene-bis-Cyclen (Cyclen = 1,4,7,10-tetraazacyclododecane) compound (tbmce) was designed with an imposed structural rigidity at the m-xylyl spacer to be compared to a less restrained and known parent compound (bmce). The framework of both compounds differs only in the substituents of the m-xylyl spacer. The study was centered in the differences observed in the acid-base reactions of both compounds, their copper(II) and zinc(II) complexation behaviors, as well as in the uptake of phosphate and polyphosphate anions (HPPi(3-), ATP(4-), ADP(3-), AMP(2-), PhPO4(2-), and HPO4(2-)). On the one hand, the acid-base reactions showed lower values for the third and fourth protonation constants of tbmce than for bmce, suggesting that the ethyl groups of the spacer in tbmce force the two Cyclen units to more conformational restricted positions. On the other hand, the stability constant values for copper(II) and zinc(II) complexes revealed that bmce is a better chelator than tbmce pointing out to additional conformational restraints imposed by the triethylbenzene spacer. The binding studies of phosphates by the dinuclear copper(II) and zinc(II) complexes showed much smaller effective association constants for the dicopper complexes. Single-crystal X-ray and computational (density functional theory) studies suggest that anion binding promotes the formation of tetranuclear entities in which anions are bridging the metal centers. Our studies also revealed the dinuclear zinc(II) complex of bmce as a promising receptor for phosphate anions, with the largest effective association constant of 5.94 log units being observed for the formation of [Zn2bmce(HPPi)](+). Accordingly, a colorimetric study via an indicator displacement assay to detect phosphates in aqueous solution found that the [Zn2bmce](4+) complex acts as the best receptor for pyrophosphate displaying a detection limit of 2.5 nM by changes visible to naked eye.


