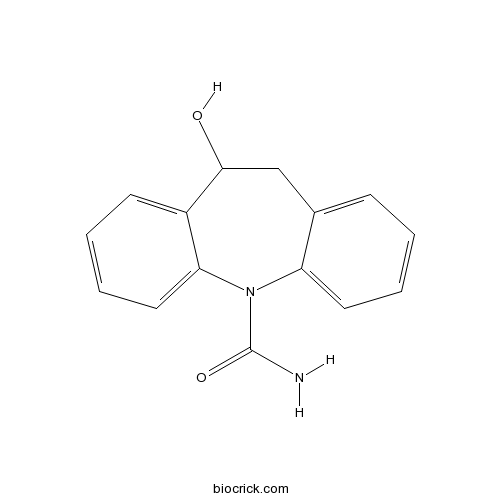
LicarbazepineCAS# 29331-92-8 |
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29331-92-8 | SDF | Download SDF |
| PubChem ID | 114709 | Appearance | Powder |
| Formula | C15H14N2O2 | M.Wt | 254.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GP 47779 | ||
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 5-hydroxy-5,6-dihydrobenzo[b][1]benzazepine-11-carboxamide | ||
| SMILES | C1C(C2=CC=CC=C2N(C3=CC=CC=C31)C(=O)N)O | ||
| Standard InChIKey | BMPDWHIDQYTSHX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14N2O2/c16-15(19)17-12-7-3-1-5-10(12)9-14(18)11-6-2-4-8-13(11)17/h1-8,14,18H,9H2,(H2,16,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Active metabolite of oxcarbazepine. Produces dose-dependent inhibition of glutamatergic excitatory postsynaptic potentials (EPSPs). Displays anticonvulsant activity; exhibits minor potentiation of GABAA receptor currents. |

Licarbazepine Dilution Calculator

Licarbazepine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9327 mL | 19.6634 mL | 39.3267 mL | 78.6535 mL | 98.3168 mL |
| 5 mM | 0.7865 mL | 3.9327 mL | 7.8653 mL | 15.7307 mL | 19.6634 mL |
| 10 mM | 0.3933 mL | 1.9663 mL | 3.9327 mL | 7.8653 mL | 9.8317 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7865 mL | 1.5731 mL | 1.9663 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9832 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
A new enantioselective CE method for determination of oxcarbazepine and licarbazepine after fungal biotransformation.[Pubmed:24981999]
Electrophoresis. 2014 Oct;35(19):2877-84.
The present work describes, for the first time, the simultaneous separation of oxcarbazepine (OXC) and its active metabolite 10-hydroxy-10,11-dihydrocarbamazepine (Licarbazepine, Lic) by chiral CE. The developed method was employed to monitor the enantioselective biotransformation of OXC into its active metabolite by fungi. The electrophoretic separations were performed using 10 mmol/L of a Tris-phosphate buffer solution (pH 2.5) containing 1% w/v of beta-CD phosphate sodium salt (P-beta-CD) as running electrolyte, -20 kV of applied voltage and a 15 degrees C capillary temperature. The method was linear over the concentration range of 1000-30 000 ng/mL for OXC and 75-900 ng/mL for each Lic enantiomer (r >/= 0.9952). Within-day precision and accuracy evaluated by RSD and relative errors, respectively, were lower than 15% for all analytes. The validated method was used to evaluate the enantioselective biotransformation of OXC, mediated by fungi, into its active metabolite Lic. This study showed that the fungi Glomerella cingulata (VA1) and Beuveria bassiana were able to enantioselectively metabolize the OXC into Lic after 360 h of incubation. Biotransformation by the fungus Beuveria bassiana showed 79% enantiomeric excess for (S)-(+)-Lic, while VA1 gave an enantiomeric excess of 100% for (S)-(+)-Lic. This study opens a new route to the drug (S)-(+)-Licarbazepine.
Oxcarbazepine and its active metabolite, (S)-licarbazepine, exacerbate seizures in a mouse model of genetic generalized epilepsy.[Pubmed:25489632]
Epilepsia. 2015 Jan;56(1):e6-9.
Oxcarbazepine (OXC), widely used to treat focal epilepsy, is reported to exacerbate seizures in patients with generalized epilepsy. OXC is metabolized to monohydroxy derivatives in two enantiomeric forms: (R)-Licarbazepine and (S)-Licarbazepine. EsLicarbazepine acetate is a recently approved antiepileptic drug that is rapidly metabolized to (S)-Licarbazepine. It is not known whether (S)-Licarbazepine exacerbates seizures. Here, we test whether OXC or either of its enantiomers exacerbates the number of spike-and-wave discharges (SWDs) in mice harboring the human gamma-aminobutyric acid A receptor (GABAA)gamma2(R43Q) mutation. OXC (20 mg/kg), (S)-Licarbazepine (20 mg/kg), and (R)-Licarbazepine (20 mg/kg) all significantly increased the number of SWDs, while their duration was unaffected. The potential for (S)-Licarbazepine to exacerbate SWDs suggests that esLicarbazepine acetate should be used with caution in generalized epilepsy. Furthermore, generalized seizure exacerbation for first-, second-, and third-generation carbamazepine-based compounds is likely to occur through a common mechanism.
Development and validation of a reversed-phase HPLC method for licarbazepine monitoring in serum of patients under oxcarbazepine treatment.[Pubmed:28182284]
Biomed Chromatogr. 2017 Sep;31(9).
Licarbazepine is the pharmacologically active metabolite of oxcarbazepine, a drug indicated for the treatment of partial seizures and bipolar disorders. Several HPLC methods have been developed thus far but there is lack of control for interferences from antipsychotic drugs. The aim of the present study was to develop a simple, low-cost and reliable HPLC-UV method for the determination of Licarbazepine in human serum in the presence of co-administered antiepileptic, antipsychotic and commonly prescribed drugs. Sample preparation consisted of a single protein precipitation step with methanol. Separation lasted ~9 min on a reversed-phase C18 column using a mobile phase composed of 50 mm sodium-dihydrogen-phosphate-monohydrate/acetonitrile (70:30, v/v) delivered isocratically at 0.9 mL/min and 30 degrees C. Wavelength was 210 nm and calibration curve was linear with r(2) 0.998 over the range 0.2-50.0 mug/mL. Coefficient of variation was <5.03% and bias <-4.92%. Recovery ranged from 99.49 to 104.52% and the limit of detection was 0.0182 mug/mL. No interferences from the matrix or from antiepileptic, antipsychotic and commonly prescribed drugs were observed. The method was applied to serum samples of patients under oxcarbazepine treatment and proved to be a useful tool for the therapeutic drug monitoring of Licarbazepine during monotherapy or adjunctive treatment of seizures or affective disorders.
Liquid chromatographic assay based on microextraction by packed sorbent for therapeutic drug monitoring of carbamazepine, lamotrigine, oxcarbazepine, phenobarbital, phenytoin and the active metabolites carbamazepine-10,11-epoxide and licarbazepine.[Pubmed:25261836]
J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Nov 15;971:20-9.
A new, sensitive and fast high-performance liquid chromatography-diode-array detection assay based on microextraction by packed sorbent (MEPS/HPLC-DAD) is herein reported, for the first time, to simultaneously quantify carbamazepine (CBZ), lamotrigine (LTG), oxcarbazepine (OXC), phenobarbital (PB), phenytoin (PHT), and the active metabolites carbamazepine-10,11-epoxide (CBZ-E) and Licarbazepine (LIC) in human plasma. Chromatographic separation of analytes and ketoprofen, used as internal standard (IS), was achieved in less than 15min on a C18-column, at 35 degrees C, using acetonitrile (6%) and a mixture (94%) of water-methanol-triethylamine (73.2:26.5:0.3, v/v/v; pH 6.5) pumped at 1mL/min. The analytes and IS were detected at 215, 237 or 280nm. The method showed to be selective, accurate [bias +/-14.8% (or +/-17.8% in the lower limit of quantification)], precise [coefficient variation /=0.9946) over the concentration ranges of 0.1-15mug/mL for CBZ; 0.1-20mug/mL for LTG; 0.1-5mug/mL for OXC and CBZ-E; 0.2-40mug/mL for PB; 0.3-30mug/mL for PHT; and 0.4-40mug/mL for LIC. The absolute extraction recovery of the analytes ranged from 57.8 to 98.1% and their stability was demonstrated in the studied conditions. This MEPS/HPLC-DAD assay was successfully applied to real plasma samples from patients, revealing to be a cost-effective tool for routine therapeutic drug monitoring of CBZ, LTG, OXC, PB and/or PHT.
Oxcarbazepine, not its active metabolite, potentiates GABAA activation and aggravates absence seizures.[Pubmed:18717705]
Epilepsia. 2009 Jan;50(1):83-7.
PURPOSE: Studies in genetic absence epileptic rats from Strasbourg (GAERS) indicate that enhancement of gamma aminobutyric acid (GABA(A)) receptor activity is a critical mechanism in the aggravation of seizures by carbamazepine (CBZ). We examined whether structural analogs of CBZ, oxcarbazepine (OXC), and its active metabolite, monohydroxy derivative (MHD), also potentiate GABA(A) receptor current and aggravate seizures. METHODS: In vitro studies in Xenopus oocytes compared the three drugs' effect on GABA(A) receptor currents. In vivo studies compared seizure activity in GAERS after intraperitoneal drug administration. RESULTS: OXC potentiated GABA(A) receptor current and aggravated seizures in GAERS, similarly to the effect of CBZ. Conversely, MHD showed only a minor potentiation of GABA(A) receptor current and did not aggravate seizures. DISCUSSION: A hydroxyl group at the C-10 position on the CBZ tricyclic structure in MHD reduces GABA(A) receptor potentiation and seizure aggravation. Reports of the aggravation of absence seizures in patients taking OXC may result from circulating unmetabolized OXC rather than MHD.
Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide derivatives.[Pubmed:10411478]
J Med Chem. 1999 Jul 15;42(14):2582-7.
A series of esters of the major metabolite of oxcarbazepine (2), 10, 11-dihydro-10-hydroxy-5H-dibenz[b,f]azepine-5-carboxamide, were synthesized and evaluated for their anticonvulsant and brain sodium channel-blocking properties. The compounds were assayed intraperitoneally and per os in rats against seizures induced by maximal electroshock (MES). Neurologic deficit was evaluated by the rotarod test. The enantiomeric acetates (R)-11 and (S)-12 were the most active of the series against MES-induced seizures with oral ED(50) values at t(max) of 10.9 +/- 2.3 and 4.7 +/- 0.9 mg/kg, respectively. After intraperitoneal administration, carbamazepine (1) behaved more potently than 2 and all other new dibenz[b, f]azepine-5-carboxamide derivatives in the MES test; compounds 2 and 12 were equally potent. In the rotarod test, low doses of 1 produced considerable motor impairment, which did not occur with 2, enantiomeric alcohols (S)-6, (R)-7, and racemic alcohol 8, or racemic acetate 10 or (R)-11. The potencies of the racemic and enantiomerically pure alcohols 8, (S)-6, and (R)-7 derived from 2 in the MES and rotarod test were found to be similar between them, and consequently they exhibit similar protective index values. All three forms of the alcohol and their corresponding acetates (pairs 8 & 10, 6 & 12, and 7 & 11) were found to differ in the MES or rotarod tests; the ED(50) value for (S)-6 against MES-induced seizures was nearly 3-fold that for (S)-12. The protective index also differed markedly between all stereoisomers of the alcohol and their corresponding acetates, most pronouncedly for compound (S)-12 which attained the highest value (12.5) among all compounds tested. Blockade of voltage-sensitive sodium channels was studied by investigating [(3)H]batrachotoxinin A 20-alpha-benzoate ([(3)H]BTX) binding. Acetates (R)-11 and (S)-12 were more potent than the standards 1 and 2 at inhibiting the binding of [(3)H]BTX to sodium channels and the influx of (22)Na(+) into rat brain synaptosomes. It is concluded that acetates (R)-11 and (S)-12 are not simple metabolic precursors of alcohols (R)-7 and (S)-6 in rodents but that they possess anticonvulsant and sodium channel-blocking properties in their own right.
Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. I. Modulation of corticostriatal synaptic transmission.[Pubmed:7555963]
Epilepsia. 1995 Oct;36(10):990-6.
Oxcarbazepine (OCBZ) is the keto-analogue of carbamazepine (CBZ). In humans, OCBZ is rapidly and almost completely metabolized to 10, 11-dihydro-10-hydroxy-CBZ (GP 47779), the main metabolite responsible for the drug's antiepileptic activity. The corticostriatal pathway is involved in the propagation of epileptic discharges. We characterized the electrophysiological effects of GP 47779 on striatal neurons by making intracellular recordings from corticostriatal slices. GP 47779 (3-100 microM) produced a dose-dependent inhibition of glutamatergic excitatory postsynaptic potentials (EPSPs). This effect was not coupled either with changes of the membrane potential of these cells or with alterations of their postsynaptic sensitivity to excitatory amino acids (EAA) suggesting a presynaptic site of action. GP 47779 reduced the current-evoked firing discharge only at concentrations > 100 microM. GP 47779 did not affect the presynaptic inhibitory action of adenosine, showing that presynaptic adenosine receptors were not implicated in the GP 47779-mediated reduction of corticostriatal EPSPs. Our data indicate that GP 47779 apparently acts directly on corticostriatal terminals to reduce the release of EAA, probably by inhibiting high-voltage-activated (HVA) calcium (Ca2+) currents (described in the accompanying article). The inhibitory action of GP 47779 on corticostriatal transmission may contribute to the antiepileptic effects of this drug.


