SieboldinCAS# 18777-73-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

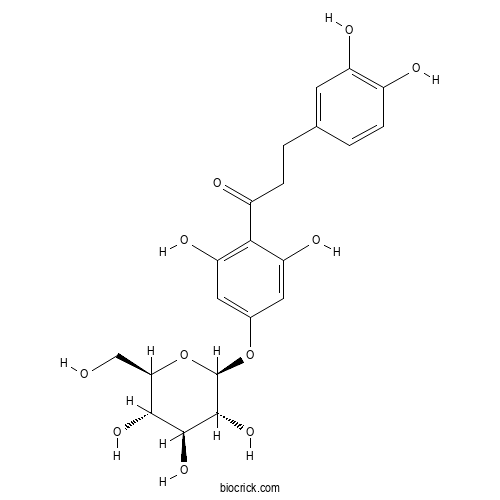
| Cas No. | 18777-73-6 | SDF | Download SDF |
| PubChem ID | 44596244 | Appearance | Powder |
| Formula | C21H24O11 | M.Wt | 452.4 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(3,4-dihydroxyphenyl)-1-[2,6-dihydroxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]propan-1-one | ||
| SMILES | C1=CC(=C(C=C1CCC(=O)C2=C(C=C(C=C2O)OC3C(C(C(C(O3)CO)O)O)O)O)O)O | ||
| Standard InChIKey | XJHMLSKQZFKMLL-QNDFHXLGSA-N | ||
| Standard InChI | InChI=1S/C21H24O11/c22-8-16-18(28)19(29)20(30)21(32-16)31-10-6-14(26)17(15(27)7-10)12(24)4-2-9-1-3-11(23)13(25)5-9/h1,3,5-7,16,18-23,25-30H,2,4,8H2/t16-,18-,19+,20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Sieboldin Dilution Calculator

Sieboldin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2104 mL | 11.0522 mL | 22.1043 mL | 44.2087 mL | 55.2608 mL |
| 5 mM | 0.4421 mL | 2.2104 mL | 4.4209 mL | 8.8417 mL | 11.0522 mL |
| 10 mM | 0.221 mL | 1.1052 mL | 2.2104 mL | 4.4209 mL | 5.5261 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.4421 mL | 0.8842 mL | 1.1052 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.4421 mL | 0.5526 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Dihydrocarvyl acetate
Catalog No.:BCN0098
CAS No.:20777-49-5
- Piperitone
Catalog No.:BCN0097
CAS No.:89-81-6
- 1,4-Anthraquinone
Catalog No.:BCN0096
CAS No.:635-12-1
- Rosmaquinone
Catalog No.:BCN0095
CAS No.:121927-71-7
- Neoarctin B
Catalog No.:BCN0094
CAS No.:155969-67-8
- 2-Methoxy-1,4-naphthoquinone
Catalog No.:BCN0093
CAS No.:2348-82-5
- (-)-Perillyl alcohol
Catalog No.:BCN0092
CAS No.:18457-55-1
- N-Formylcytisine
Catalog No.:BCN0091
CAS No.:53007-06-0
- Fumarprotocetraric acid
Catalog No.:BCN0090
CAS No.:489-50-9
- Serpentine hydrogen tartrate
Catalog No.:BCN0089
CAS No.:58782-36-8
- 2,4,6-Trihydroxybenzoic acid
Catalog No.:BCN0088
CAS No.:83-30-7
- Quercetin 3,5,7,3,4-pentamethyl ether
Catalog No.:BCN0087
CAS No.:1247-97-8
- Heptyl acetate
Catalog No.:BCN0100
CAS No.:112-06-1
- 3,6-Dihydroxyflavone
Catalog No.:BCN0101
CAS No.:108238-41-1
- trans-2-Hexen-1-al
Catalog No.:BCN0102
CAS No.:6728-26-3
- trans-2-Hexen-1-ol
Catalog No.:BCN0103
CAS No.:928-95-0
- N-Malonyl DL-tryptophan
Catalog No.:BCN0104
CAS No.:3184-74-5
- Rigosertib (ON-01910)
Catalog No.:BCN0105
CAS No.:1225497-78-8
- Physalien
Catalog No.:BCN0106
CAS No.:144-67-2
- 1-Heptacosanol
Catalog No.:BCN0107
CAS No.:2004-39-9
- 3-Hydroxybenzaldehyde
Catalog No.:BCN0108
CAS No.:100-83-4
- DL-Threonine
Catalog No.:BCN0109
CAS No.:80-68-2
- Furfuryl acetate
Catalog No.:BCN0110
CAS No.:623-17-6
- Eupatorin-5-methylether
Catalog No.:BCN0111
CAS No.:21764-09-0
Biosynthesis of the Dihydrochalcone Sweetener Trilobatin Requires Phloretin Glycosyltransferase2.[Pubmed:32732350]
Plant Physiol. 2020 Oct;184(2):738-752.
Epidemics of obesity and type 2 diabetes drive strong consumer interest in plant-based low-calorie sweeteners. Trilobatin is a sweetener found at high concentrations in the leaves of a range of crabapple (Malus) species, but not in domesticated apple (Malus x domestica) leaves, which contain trilobatin's bitter positional isomer phloridzin. Variation in trilobatin content was mapped to the Trilobatin locus on LG 7 in a segregating population developed from a cross between domesticated apples and crabapples. Phloretin glycosyltransferase2 (PGT2) was identified by activity-directed protein purification and differential gene expression analysis in samples high in trilobatin but low in phloridzin. Markers developed for PGT2 cosegregated strictly with the Trilobatin locus. Biochemical analysis showed PGT2 efficiently catalyzed 4'-o-glycosylation of phloretin to trilobatin as well as 3-hydroxyphloretin to Sieboldin. Transient expression of double bond reductase, chalcone synthase, and PGT2 genes reconstituted the apple pathway for trilobatin production in Nicotiana benthamiana Transgenic M. x domestica plants overexpressing PGT2 produced high concentrations of trilobatin in young leaves. Transgenic plants were phenotypically normal, and no differences in disease susceptibility were observed compared to wild-type plants grown under simulated field conditions. Sensory analysis indicated that apple leaf teas from PGT2 transgenics were readily discriminated from control leaf teas and were perceived as significantly sweeter. Identification of PGT2 allows marker-aided selection to be developed to breed apples containing trilobatin, and for high amounts of this natural low-calorie sweetener to be produced via biopharming and metabolic engineering in yeast.
Metazoan Parasite Faunas of Three Gobiid Species (Actinopterygii: Gobiidae) Inhabiting the Lower Kizilirmak Delta in Samsun: A Comparative Study.[Pubmed:29780017]
Turkiye Parazitol Derg. 2018 Mar;42(1):33-38.
OBJECTIVE: The aim of the present study was to investigate and compare metazoan parasite faunas of three gobiid fishes, Neogobius fluviatilis, Proterorhinus marmoratus, and Pomatoschistus marmoratus, inhabiting the Lower Kizilirmak Delta. METHODS: Fish specimens were caught using fishing nets and electroshock device. The fishes were transferred to the laboratory and examined under a dissecting microscope for metazoan parasites using conventional methods. The isolated parasites were fixed with 70% ethyl alcohol. The Czekanowski-Sorensen Index (ICS) was used for comparing the metazoan faunas of the three gobiid fishes. RESULTS: Overall, 13 metazoan parasite species comprising 2 monogeneans (Gyrodactylus proterorhini and Gyrodactylus sp.), 6 digenean metacercariae (Tylodelphys clavata, Diplostomum spathaceum, Apatemon gracilis, Posthodiplostomum sp., Ascocotyle sp., and Echinostoma sp.), 1 cestoda (Bothriocephalus acheilognathi), 3 nematodes (Spiroxys contortus, Eustrongylides excisus, and Contraceacum rudolphii), and 1 arthropoda Ergasilus (Sieboldin) were observed. The maximum parasite diversity was found in N. fluviatilis; Po. marmoratus had significantly fewer parasitic species (4). Total parasite abundance was significantly high in Pr. marmoratus, which was infected with 9 parasite species. A closer resemblance was observed in the parasite faunas of N. fluviatilis and Pr. marmoratus (ICS=80.0%). CONCLUSIONS: To the best of our knowledge, this is the first study on metazoan parasite faunas of N. fluviatilis, Pr. marmoratus, and Po. marmoratus in Turkey.
Analysis of Phenolic Compounds in Rooibos Tea (Aspalathus linearis) with a Comparison of Flavonoid-Based Compounds in Natural Populations of Plants from Different Regions.[Pubmed:29063755]
J Agric Food Chem. 2017 Nov 29;65(47):10270-10281.
Tea samples from 17 populations of "wild tea" ecotypes Aspalathus linearis (rooibos tea) and 2 populations of Aspalathus pendula were analyzed. Recent advances in column technology together with high-resolution mass spectrometry were applied to improve resolution, facilitating the identification of several new compounds as well as grouping of the wild tea ecotypes according to their chemical composition. The collisional cross-section data obtained from ion mobility-mass spectrometry is reported for the flavonoids in rooibos for the first time. Enzyme pathways for the synthesis of the unique flavonoids found in rooibos tea are also proposed. A. linearis and A. pendula produce similar combinations of main phenolic compounds, with no diagnostically different discontinuities between populations or species. Northern resprouters (Gifberg and Nieuwoudtville) contain higher phenylpropenoic acid glucoside levels while teas from Wupperthal and surrounding areas were found to contain unique dihydrochalcones (phloridzin and a Sieboldin analog), which are reported here for the first time.
Extraction, identification, and antioxidant and anticancer tests of seven dihydrochalcones from Malus 'Red Splendor' fruit.[Pubmed:28450013]
Food Chem. 2017 Sep 15;231:324-331.
Five dihydrochalcone compounds, including phlorizin, trilobatin, 3-hydroxyphlorizin, Sieboldin and phloretin 2'-xyloglucoside, were isolated from ornamental Malus 'Red Splendor' fruit. The chemical structures of these compounds were elucidated by LC-ESI-MS and NMR. Phloretin and 3-hydroxyphloretin were produced by hydrolysis. The antioxidant capacities of these seven compounds were examined by DPPH and ABTS assays, while their cytotoxicity to five cancer cell lines were evaluated by the MTT assay. The results showed that the DPPH assay mainly reflected the antioxidant capacity of the B ring, whereas the ABTS assay was mostly related to the A ring of the dihydrochalcone molecule. Moreover, 3-hydroxyphloretin was the best antioxidant among the seven compounds. Both glycosylation of the A ring and the ortho phenolic hydroxyl groups of the B ring were important for the cytotoxicity of dihydrochalcone molecules. Sieboldin and 3-hydroxyphlorizin exhibited better cytotoxicity than other dihydrochalcone compounds. Dihydrochalcones from Malus may benefit human health.
A new phenolic constituent and a cyanogenic glycoside from Balanophora involucrata (Balanophoraceae).[Pubmed:23776023]
Chem Biodivers. 2013 Jun;10(6):1081-7.
Balanophora involucrata HOOK.f. & THOMSON (Balanophoraceae) is a parasite plant often growing on the roots of leguminous plants. The whole herb has been used medicinally for the treatment of irregular menstruation, cough, hemoptysis, traumatic injury and bleeding, dizziness and gastralgia in Yunnan Province, China. The 2,2-diphenyl-2-picrylhydrazyl (DPPH) assay on the 60% aq. acetone extract of the fresh whole plant of B. involucrata showed considerable radical-scavenging activity (SC(5)(0) 15.3 mug/ml). Further purification on the extract led to the isolation of one new phenolic glycoside, Sieboldin-3'-ketocarboxylic acid (1), and one new cyanogenic glycoside, proacacipetalin 6'-O-beta-D-glucopyranoside (2), together with 26 known compounds including three 4"-O-galloyl and 2",3"-O-(S)-hexahydroxydiphenoyl (HHDP) derivatives of dihydrochalcone glucosides, seven hydrolyzable tannins, and alkane glycosides. The cyanogenic compound isolated from the Balanophoraceae family for the first time might be a signal molecule between B. involucrata and its hosts. The free-radical-scavenging activity of the isolated compounds was also examined by DPPH assay.
Histolocalization and physico-chemical characterization of dihydrochalcones: Insight into the role of apple major flavonoids.[Pubmed:23562371]
Phytochemistry. 2013 Jun;90:78-89.
Flavonoids, like other metabolites synthesized via the phenylpropanoid pathway, possess a wide range of biological activities including functions in plant development and its interaction with the environment. Dihydrochalcones (mainly phloridzin, Sieboldin, trilobatin, phloretin) represent the major flavonoid subgroup in apple green tissues. Although this class of phenolic compounds is found in very large amounts in some tissues ( approximately 200mg/g of leaf DW), their physiological significance remains unclear. In the present study, we highlight their tissue-specific localization in young growing shoots suggesting a specific role in important physiological processes, most notably in response to biotic stress. Indeed, dihydrochalcones could constitute a basal defense, in particular phloretin which exhibits a strong broad-range bactericidal and fungicidal activity. Our results also indicate that Sieboldin forms complexes with iron with strong affinity, reinforcing its antioxidant properties and conferring to this dihydrochalcone a potential for iron seclusion and/or storage. The importance of localization and biochemical properties of dihydrochalcones are discussed in view of the apple tree defense strategy against both biotic and abiotic stresses.
Dihydrochalcones: Implication in resistance to oxidative stress and bioactivities against advanced glycation end-products and vasoconstriction.[Pubmed:20022617]
Phytochemistry. 2010 Mar;71(4):443-52.
Flavonoids are a group of polyphenol compounds with known antioxidant activities. Among them, dihydrochalcones are mainly found in apple leaves (Malus domestica). Glycosylated dihydrochalcones were previously found in large amounts in leaves of two genotypes of Malus with contrasting resistance to fire blight, a bacterial disease caused by Erwinia amylovora. In the present study we demonstrate that soluble polyphenol patterns comprised phloridzin alone or in combination with two additional dihydrochalcones, identified as Sieboldin and trilobatin. Presence of Sieboldin in young leaves correlated well with a high 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. Moreover, these leaves displayed enhanced tolerance to paraquat, a photooxidative-stress generating herbicide. Interestingly, phloridzin had a high activity in the oxygen radical absorbance capacity (ORAC) assay, but its presence alone in leaves did not correlate with tolerance to paraquat. In order to further characterise the activity of these compounds, we tested their ability to prevent oxidative-dependent formation of advanced glycation end-products (AGEs) and phenylephrine-induced contraction of isolated rat mesenteric arteries. The antioxidant capacity of Sieboldin was clearly demonstrated by showing that this compound (i) prevented vasoconstriction and (ii) inhibited AGEs formation. Both assays provided interesting information concerning a potential use of Sieboldin as a therapeutic. Hence, our results strongly argue for a bioactivity of dihydrochalcones as functional antioxidants in the resistance of Malus leaves to oxidative stress. In addition, we demonstrate for the first time that Sieboldin is a powerful multipotent antioxidant, effective in preventing physiopathological processes. Further work should aim at demonstrating the potential use of this compound as a therapeutic in treating free radical-involving diseases.
Characterization and cDNA cloning of monomeric lectins that correspond to the B-Chain of a type 2 ribosome-inactivating protein from the bark of Japanese elderberry (Sambucus sieboldiana).[Pubmed:15115776]
J Biochem. 2004 Apr;135(4):509-16.
Two monomeric lectins, SSA-b-3 and SSA-b-4, were purified from the bark tissue of Japanese elderberry, Sambucus sieboldiana. SDS-PAGE of the purified lectins showed the presence of single bands of 35 and 33 kDa for SSA-b-3 and SSA-b-4, respectively, irrespective of the presence of reducing agent. MS analysis as well as gel filtration of these lectins indicated that they exist mostly as monomeric lectins. Analysis of the N-terminal amino acid sequences of SSA-b-3 and SSA-b-4 yielded an identical sequence, indicating their close structural relationship. Four cDNA clones with extensive homology were obtained from the bark cDNA library and indicated to encode SSA-b-3 or SSA-b-4 from the comparison with the N-terminal sequences of these lectins. These clones were classified into two groups, three for SSA-b-3 and one for SSA-b-4, based on the predicted isoelectric points. The amino acid sequences of the encoded polypeptides were almost identical with the B-chain of a type 2 ribosome-inactivating protein from the same bark tissue, Sieboldin-b, except for the absence of a small peptide containing a cystein residue, which is critical for the heteromeric dimerization with an A-subunit. Carbohydrate binding specificity and biological activity of these lectins are also reported.
Isolation, cDNA cloning, biological properties, and carbohydrate binding specificity of sieboldin-b, a type II ribosome-inactivating protein from the bark of Japanese elderberry (Sambucus sieboldiana).[Pubmed:9143320]
Arch Biochem Biophys. 1997 Apr 15;340(2):185-94.
A type II ribosome-inactivating protein (RIP) was isolated from the bark tissue of Japanese elderberry (Sambucus sieboldiana) and named Sieboldin-b. Sieboldin-b is a heterodimeric protein consisting of 27- and 33-kDa subunits and showed strong ribosome-inactivating activity in vitro but did not show in vivo toxicity. The amino acid sequence of Sieboldin-b deduced from the structure of the cDNA showed that both subunits of Sieboldin-b are encoded on a single precursor polypeptide. Sieboldin-b has a structure homologous with the Neu5Ac(alpha 2-6)Gal/GalNAc-specific bark lectin from S. sieboldiana (SSA) and also typical type II RIPs such as ricin and abrin. Detailed analyses of carbohydrate binding properties of Sieboldin-b revealed that Sieboldin-b binds to Gal/GalNAc, similar to ricin/abrin, in spite of its highly homologous structure with SSA. The biological properties of these toxins/lectins are compared, and the possible explanation for such diversity is discussed.


