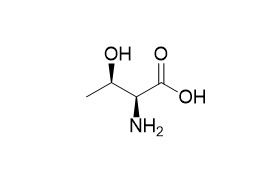
DL-ThreonineCAS# 80-68-2 |
- Allo-Thr-OH
Catalog No.:BCC3101
CAS No.:28954-12-3
- H-D-Thr-OH
Catalog No.:BCC3108
CAS No.:632-20-2
- H-Thr-OH
Catalog No.:BCC3102
CAS No.:72-19-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80-68-2 | SDF | Download SDF |
| PubChem ID | 205 | Appearance | Powder |
| Formula | C4H9NO3 | M.Wt | 119.1 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-amino-3-hydroxybutanoic acid | ||
| SMILES | CC(C(C(=O)O)N)O | ||
| Standard InChIKey | AYFVYJQAPQTCCC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H9NO3/c1-2(6)3(5)4(7)8/h2-3,6H,5H2,1H3,(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DL-Threonine has nutritional effects. DL-Threonine does not contribute to the healing of blister bases but that it is of benefit in hypostatic leg ulceration. | |||||

DL-Threonine Dilution Calculator

DL-Threonine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.3963 mL | 41.9815 mL | 83.9631 mL | 167.9261 mL | 209.9076 mL |
| 5 mM | 1.6793 mL | 8.3963 mL | 16.7926 mL | 33.5852 mL | 41.9815 mL |
| 10 mM | 0.8396 mL | 4.1982 mL | 8.3963 mL | 16.7926 mL | 20.9908 mL |
| 50 mM | 0.1679 mL | 0.8396 mL | 1.6793 mL | 3.3585 mL | 4.1982 mL |
| 100 mM | 0.084 mL | 0.4198 mL | 0.8396 mL | 1.6793 mL | 2.0991 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Hydroxybenzaldehyde
Catalog No.:BCN0108
CAS No.:100-83-4
- 1-Heptacosanol
Catalog No.:BCN0107
CAS No.:2004-39-9
- Physalien
Catalog No.:BCN0106
CAS No.:144-67-2
- Rigosertib (ON-01910)
Catalog No.:BCN0105
CAS No.:1225497-78-8
- N-Malonyl DL-tryptophan
Catalog No.:BCN0104
CAS No.:3184-74-5
- trans-2-Hexen-1-ol
Catalog No.:BCN0103
CAS No.:928-95-0
- trans-2-Hexen-1-al
Catalog No.:BCN0102
CAS No.:6728-26-3
- 3,6-Dihydroxyflavone
Catalog No.:BCN0101
CAS No.:108238-41-1
- Heptyl acetate
Catalog No.:BCN0100
CAS No.:112-06-1
- Sieboldin
Catalog No.:BCN0099
CAS No.:18777-73-6
- (-)-Dihydrocarvyl acetate
Catalog No.:BCN0098
CAS No.:20777-49-5
- Piperitone
Catalog No.:BCN0097
CAS No.:89-81-6
- Furfuryl acetate
Catalog No.:BCN0110
CAS No.:623-17-6
- Eupatorin-5-methylether
Catalog No.:BCN0111
CAS No.:21764-09-0
- Dehydroascorbic acid
Catalog No.:BCN0112
CAS No.:490-83-5
- alpha-Pinene oxide
Catalog No.:BCN0113
CAS No.:1686-14-2
- (+)-Isocorydine hydrochloride
Catalog No.:BCN0114
CAS No.:13552-72-2
- Sempervirine nitrate
Catalog No.:BCN0115
CAS No.:17994-15-9
- (-)-Carveol
Catalog No.:BCN0116
CAS No.:99-48-9
- Tricetinidin chloride
Catalog No.:BCN0117
CAS No.:65618-21-5
- Lactupicrin
Catalog No.:BCN0118
CAS No.:65725-11-3
- VX-702
Catalog No.:BCN0119
CAS No.:479543-46-9
- (+)-Longifolene
Catalog No.:BCN0120
CAS No.:475-20-7
- k-Strophanthoside
Catalog No.:BCN0121
CAS No.:33279-57-1
Shortcut Model for Describing Isothermal Batch Preferential Crystallization of Conglomerates and Estimating the Productivity.[Pubmed:32952449]
Cryst Growth Des. 2019 Sep 4;19(9):5189-5203.
Preferential crystallization (PC) is a powerful method to separate the enantiomers of chiral molecules that crystallize as conglomerates. The kinetically controlled separation method works in a typically narrow metastable zone. Currently, there are no simple models available that allow estimating the productivity of PC and, thus, the comparison with rivalling resolution techniques. In this Article, we suggest a simple shortcut model (SCM) capable of describing the main features of batch-wise operated PC using three ordinary differential equations originating from the mass balance of the target enantiomer and solvent in the liquid and solid phases. Compared to population balance models, the basis of the SCM is the assumption that the crystals for each enantiomer have the same size, which increases continuously from prespecified initial values. The goal of the model is to describe the initial period of the batch, during which the purity is within the specification required. It is accepted that after reaching this border, the precision of predictions can drop. This Article also illustrates a simple strategy how to parametrize the model based on a few experimental runs of PC. At first, for demonstration purposes, theoretical transients generated using the more rigorous PBE model is analyzed using SCM considering the separation of the enantiomers of DL-Threonine. Subsequently, results of an experimental study with the enantiomers of asparagine monohydrate are presented to validate the shortcut model, which is seen as a new valuable tool to quantify more rapidly the productivity of PC and to further promote this elegant technique capable to resolve enantiomers of conglomerate forming chiral systems.
Gimesia chilikensis sp. nov., a haloalkali-tolerant planctomycete isolated from Chilika lagoon and emended description of the genus Gimesia.[Pubmed:32369005]
Int J Syst Evol Microbiol. 2020 Jun;70(6):3647-3655.
A Gram-stain-negative, aerobic, non-motile, salt- and alkali-tolerant, pear to oval shaped, rosette-forming, white coloured, bacterium, designated as strain JC646(T), was isolated from a sediment sample collected from Chilika lagoon, India. Strain JC646(T) reproduced through budding, grew well at up to pH 9.0 and tolerated up to 7 % NaCl. Strain JC 646(T) utilized alpha-d-glucose, fumarate, lactose, sucrose, fructose, d-galactose, mannose, maltose and d-xylose as carbon sources. Peptone, l-isoleucine, l-serine, l-lysine, l-glutamic acid, l-aspartic acid, DL-Threonine and l-glycine were used by the strain as nitrogen sources for growth. The respiratory quinone was MK6. Major fatty acids were C16 : 1 omega7c/C16 : 1 omega6c and C16 : 0. The polar lipids of strain JC646(T) comprised phosphatidyl-dimethylethanolamine, phosphatidylcholine, diphosphatidylglycerol, an unidentified amino lipid and two unidentified lipids. Strain JC646(T) had highest (97.3 %) 16S rRNA gene sequence identity to the only species of the genus Gimesia, Gimesia maris DSM 8797(T). The genome of strain JC646(T) was 7.64 Mbp with a DNA G+C content of 53.2 mol%. For the resolution of the phylogenetic congruence of the novel strain, the phylogeny was also reconstructed with the sequences of 92 housekeeping genes. Based on phylogenetic analyses, digital DNA-DNA hybridization (19.0 %), genome average nucleotide identity (74.5 %) and average amino acid identity/percentageof conserved proteins (77 %) results, chemotaxonomic characteristics, and differential physiological properties, strain JC646(T) is recognized as representing a new species of the genus Gimesia, for which we propose the name Gimesia chilikensis sp. nov. The type strain is JC646(T) (=KCTC 72175(T)=NBRC 113881(T)).
Special features of monolayer characteristics of N-alkanoyl substituted threonine amphiphiles.[Pubmed:30519690]
Phys Chem Chem Phys. 2018 Dec 19;21(1):96-103.
The monolayers of N-alkanoyl substituted threonine amphiphiles, similar to those of other N-alkanoyl-substituted amino acid amphiphiles, point to substantial differences in the main characteristics compared to usual amphiphilic monolayers. pi-A measurements of the enantiomeric and racemic forms of N-alkanoyl-substituted threonine monolayers with C16 and C18 chain lengths reveal that, independent of the alkyl chain length, all compression curves are located above the corresponding decompression curves. A theoretical model developed for the kinetics of two-dimensional condensation of Langmuir monolayers can describe this behavior concluding the attachment of monomers to large aggregates. The linear fit of the entropy changes versus temperature (DeltaS = f(T)) at the LE/LC phase transition and extrapolation to zero DeltaS specifies the critical temperature Tc, above which the monolayer cannot be compressed into the condensed state. The relatively small DeltaTc difference between the enantiomeric and the racemic forms is consistent with the increased strength of van der Waals interactions between the longer alkyl chains reducing the influence of chirality on the thermodynamic parameters. The BAM experiments reveal clearly the absence of inner anisotropy as a specific feature of the domain topology of N-palmitoyl-threonine monolayers. Furthermore, the growth kinetics of the racemic N-palmitoyl-DL-Threonine domains reveals a transition from homochiral discrimination and chiral separation within the domain to a state with heterochiral preference. GIXD studies show that at all pressures the enantiomers exhibit three Bragg peaks indicating an oblique lattice structure, whereas the racemates show only two Bragg peaks indicating a NNN tilted orthorhombic structure. Characteristic for the structure of all condensed monolayer phases is the large tilt angle of approximately 49 degrees , nearly independent of the lateral pressure. The transition from the oblique lattice structures, as detected for enantiomeric monolayers, to orthorhombic structures of racemic monolayers is clear evidence that the dominant heterochiral interaction in the racemic mixtures leads to the formation of a compound with congruent transition pressure having with approximately 20.0 A2 an essentially smaller alkyl chain cross-sectional area than the enantiomers with approximately 20.7 A2.
Determination of Hepatoma-Associated DL-Amino Acids Enantiomers by RP-HPLC with Fluorescence Detector: Application in Patients with Hepatocellular Carcinoma.[Pubmed:30143491]
Ann Clin Lab Sci. 2018 Jul;48(4):490-495.
BACKGROUND: Amino acids are increasingly being recognized as important signaling molecules in the pathogenesis of many diseases. We aim to establish a reversed-phase high-performance liquid chromatography with fluorescence detector (RP-HPLC-FLD) method for determination and quantification of hepatoma-associated DL-amino acids and to explore the relationship between amino acid concentrations and hepatocellular carcinoma (HCC). METHODS: In this work, O-phthaldialdehyde (OPA) and N-isobutyryl-L-cysteine (NAC) served as the pre-column derivatization reagents which significantly shortened the detection time and improved the detection sensitivity. Chromatographic determination was achieved using a programmed gradient elution with a flow rate of 1.0 mL/min. The eluted solution was monitored by a fluorescence detector with an excitation wavelength at 350 nm and an emission wavelength at 450 nm. Under the optimum conditions, an excellent quantification of DL-Threonine, alanine, tyrosine, valine, methionine and phenylalanine was achieved. RESULTS: Total analysis time was shortened to less than 25 min for one plasma sample and the linearity, recovery, intra- and inter-day precision were all meet the detection requirements of the DL-amino acids enantiomers in human plasma samples. CONCLUSIONS: The developed method demonstrates that DL-Threonine, alanine, tyrosine, valine and methionine obtained from HCC patients' plasma samples have a close relationship with HCC. The method would be a potentially alternative tool for DL-amino acids detection in clinical samples.
Crystallisation from a Water-in-Oil Emulsion as a Route to Enantiomer Separation: The Case of DL-Threonine.[Pubmed:26577229]
Chemistry. 2016 Jan 4;22(1):74-8.
The use of crystalliation as a means of separating enantiomers is well known. The utility of commonly applied seeding approaches is limited by the ultimate crystallisation of the antipode. Here we demonstrate how the combination of colloid science and crystal chemistry can lead to an emulsion based process yielding robust separation of a purified solid and impure liquid phases with ultimate product ee of up to 90 %. Threonine is used as a model to demonstrate the viability of the method but it is clear that extension to include, for example, simultaneous racemisation within the disperse phase is easily possible and would transform this from a separation to a preparation process.
Improving the electro-transformation efficiency of Corynebacterium glutamicum by weakening its cell wall and increasing the cytoplasmic membrane fluidity.[Pubmed:26354854]
Biotechnol Lett. 2015 Dec;37(12):2445-52.
OBJECTIVE: To improve the transformation efficiency of Corynebacterium glutamicum cells with heterogenous plasmid DNA and single-strand DNA (ssDNA) using a methodology based on electro-transformation. RESULTS: A semicomplex hypertonic medium was selected with addition of glycine and DL-Threonine to weaken cell walls and addition of Tween 80 and isonicotinic acid hydrazide to increase cytoplasmic membrane fluidity. Their contents were optimized by response surface methodology. Cell growth, electro-transformation buffer, and transformation protocol were also optimized. Temporary heating inactivation of the host restriction enzyme showed a significant effect. Finally, a high transformation efficiency of 3.57 +/- 0.13 x 10(7) cfu/mug DNA of plasmid and 1.05 x 10(6) Str (R) cfu per 10(9) viable cells with a ssDNA was achieved. CONCLUSION: The results shed light on the application in functional genomics and genome editing of C. glutamicum.
Plasmid transformation of Weissella paramesenteroides DX by electroporation.[Pubmed:25199701]
Anaerobe. 2014 Dec;30:60-4.
The present investigation sought to provide a reliable and highly efficient electrotransformation method for the lactic acid bacterium Weissella paramesenteroides DX. Experiments were carried out with the shuttle vectors pVS44 (2910 bps), pTRKH3 (7766 bps) and its derivative pTRKH3-1 (4855 bps). Several parameters, including the concentration of transforming plasmid DNA, plasmid size, electric field strength, age of the culture, cell density, and the pretreatment of cells with DL-Threonine, lysozyme, and combined treatment with lithium acetate and dithiothreitol, were investigated and proved to influence the efficiency of transformation. Electrocompetence was found to peak in the early stationary phase (OD600 1.2). Other optimized conditions included: the concentration of 10 mug/ml transforming DNA, the cell density of 10(10) cells/ml, a high-density electric field pulse of 2.5 kV, 25 muF and 200 Omega, pretreatment of cells with 40 mM DL-Threonine and 2000 U/ml lysozyme, and yielded 3.5x10(4) transformants/mug DNA for pVS44 while 1.2x10(4) transformants/mug DNA for the large plasmid TRKH3. Compared to previously reported data, the obtained transformation efficiencies provided an 8.75-fold increase for pVS44 and ensured plasmid stability for 120 generations in non-selective medium.
Enhanced antibiotic production by Streptomyces sindenensis using artificial neural networks coupled with genetic algorithm and Nelder-Mead downhill simplex.[Pubmed:22580313]
J Microbiol Biotechnol. 2012 Jul;22(7):939-46.
Antibiotic production with Streptomyces sindenensis MTCC 8122 was optimized under submerged fermentation conditions by artificial neural network (ANN) coupled with genetic algorithm (GA) and Nelder-Mead downhill simplex (NMDS). Feed forward back-propagation ANN was trained to establish the mathematical relationship among the medium components and length of incubation period for achieving maximum antibiotic yield. The optimization strategy involved growing the culture with varying concentrations of various medium components for different incubation periods. Under non-optimized condition, antibiotic production was found to be 95 microgram/ml, which nearly doubled (176 microgram/ml) with the ANN-GA optimization. ANN-NMDS optimization was found to be more efficacious, and maximum antibiotic production (197 microgram/ml) was obtained by cultivating the cells with (g/l) fructose 2.7602, MgSO4 1.2369, (NH4)2PO4 0.2742, DL-Threonine 3.069%, and soyabean meal 1.952%, for 9.8531 days of incubation, which was roughly 12% higher than the yield obtained by ANN coupled with GA under the same conditions.
Enhancing electro-transformation competency of recalcitrant Bacillus amyloliquefaciens by combining cell-wall weakening and cell-membrane fluidity disturbing.[Pubmed:20951110]
Anal Biochem. 2011 Feb 1;409(1):130-7.
Bacillus amyloliquefaciens has been a major workhorse for the production of a variety of commercially important enzymes and metabolites for the past decades. Some subspecies of this bacterium are recalcitrant to exogenous DNA, and transformation with plasmid DNA is usually less efficient, thereby limiting the genetic manipulation of the recalcitrant species. In this work, a methodology based on electro-transformation has been developed, in which the cells were grown in a semicomplex hypertonic medium, cell walls were weakened by adding glycine (Gly) and DL-Threonine (DL-Thr), and the cell-membrane fluidity was elevated by supplementing Tween 80. After optimization of the cell-loosening recipe by response surface methodology (RSM), the transformation efficiency reached 1.13 +/- 0.34 x 10(7) cfu/mug syngeneic pUB110 DNA in a low conductivity electroporation buffer. Moreover, by temporary heat inactivation of the host restriction enzyme, a transformation efficiency of 8.94 +/- 0.77 x 10(5) cfu/mug DNA was achieved with xenogeneic shuttle plasmids, a 10(3)-fold increase compared to that reported previously. The optimized protocol was also applicable to other recalcitrant B. amyloliquefaciens strains used in this study. This work could shed light on the functional genomics and subsequent strain improvement of the recalcitrant Bacillus, which are difficult to be transformed using conventional methods.
Effect of amino acids on tannase biosynthesis by Bacillus licheniformis KBR6.[Pubmed:19597651]
J Microbiol Immunol Infect. 2009 Apr;42(2):172-5.
BACKGROUND AND PURPOSE: Microbial tannase (tannin acyl hydrolase, EC 3.1.1.20), a hydrolysable tannin-degrading enzyme, has gained importance in various industrial processes, and is used extensively in the manufacture of instant tea, beer, wine, and gallic acid. Tannase is an inducible enzyme, and hydrolysable tannin, especially tannic acid, is the sole inducer. This study is of the effect of various amino acids and their analogues on tannase biosynthesis by Bacillus licheniformis KBR6 to ascertain the mode of action of these growth factors on tannase biosynthesis from microbial origin. METHODS: Enzyme production was carried out in enriched tannic acid medium through submerged fermentation for 20 h at 35 degrees C. Different amino acids at a concentration of 0.05 g% (w/v) were added to the culture medium immediately after sterilization. Culture supernatant was used as the source of the enzyme and the quantity of tannase was estimated by the colorimetric assay method. Growth of the organism was estimated according to biomass dry weight. RESULTS: Maximum tannase (2.87-fold that of the control) was synthesized by B. licheniformis KBR6 when alanine was added to the culture medium. Other amino acids, such as DL-serine, L-cystine, glycine, L-ornithine, aspartic acid, L-glutamic acid, DL-valine, L-leucine and L-lysine, also induced tannase synthesis. L-Cysteine monohydrochloride and DL-Threonine were the most potent inhibitors. CONCLUSIONS: Regulation of tannase biosynthesis by B. licheniformis in the presence of various amino acids is shown. This information will be helpful for formulating an enriched culture medium for industrial-scale tannase production.
Optimization of technical conditions for the transformation of Pediococcus acidilactici P60 by electroporation.[Pubmed:17275085]
Plasmid. 2007 Jul;58(1):44-50.
Previously reported techniques for the electrotransfer of foreign DNA into pediococci yield only a small number of transformants/mug DNA, especially when using undomesticated strains. This study reports an improved protocol for the electrotransformation of pediococci, based on trials using Pediococcus acidilactici P60 and the plasmid pRS4C1. The improved protocol yields from 2 to 3 log units more transformants than the previously reported methods, with up to (9.1+/-1.3)x10(4) transformants/mug of foreign DNA under the best conditions identified. The most important modifications proposed are an increase in electric field strength during electroporation (from 12.5 to 20kV/cm) and a reduction in lysozyme concentration during the preparation of electrocompetent cells (from 4000 to 2000U/ml): together, these two modifications greatly improve transformant yield. In addition, increasing cell culture time (from OD(600nm)=0.6 to OD(600nm)=1.0-1.2) and increasing DL-Threonine concentration in the growth medium (from 20 to 40mM) also contribute to improved electrotransformation efficiency.
[Synthesis and DNA binding spectroscopy studies of Cu(II)-Thr-Phen].[Pubmed:16379285]
Guang Pu Xue Yu Guang Pu Fen Xi. 2005 Sep;25(9):1439-42.
A new complex [Cu (Thr)(Phen)H2O] SO4 . HO2 x CH3 OH (Thr = DL-Threonine, Phen = o-Phenanthroline), which has not been published, was synthesized and characterized by elemental analysis, IR spectroscopy, and TG-DTA. The interaction of the complex and sperm DNA was studied by electronic absorption and ethidium bromide (EB) fluorescence spectroscopy. The result indicates that the maximal absorption peaks of this complex are red-shifted and the intensity is weakened; At the same time, it can to some extent quench the emission intensity of EB-DNA system. Therefore, the authors come to a conclusion that the interaction of this complex and sperm DNA is intercalation.
Analysis of applying different solvents for the mobile phase and for sample injection.[Pubmed:16188569]
J Chromatogr A. 2005 Oct 21;1092(1):142-8.
Overloading a chromatographic column with a compound possessing low solubility in the mobile phase has been investigated. In order to increase the concentration of injection a strong solvent for dissolving the feed was used. The injection of such concentrated samples brings the risk of triggering undesired crystallisation processes. A model system has been investigated with ethanol-water as the mobile phase and DL-Threonine as the sample dissolved in pure water. Under extreme overloaded conditions band splitting was observed. Measurements of the adsorption isotherms and systematic solubility studies were carried out. For the process analysis a simplified mathematical model was applied. The simulations of the band profiles were compared with the experimental data.
Phospholipid metabolism and protein kinase C mediated protein phosphorylation in dietary protein deficiency in rat lung.[Pubmed:16053266]
Indian J Exp Biol. 2005 Jul;43(7):606-13.
Nutritional deprivation of proteins decreases the protein kinase C (PKC) activity in rat lung. The activity of (PKC) is influenced by lipid metabolism. Changes in PKC activity may influence phosphorylation of its substrate proteins in the tissues. Therefore, alterations in phospholipid metabolism and PKC mediated protein phosphorylation in dietary protein deficiency in rat lung were envisaged. The study was conducted on rats fed on three different types of diet viz., casein (20% protein), deficient (4% protein, rice flour as source of protein) and supplemented (deficient diet supplemented with L-lysine and DL-threoning). Feeding of protein deficient diet caused reduction in incorporation of [3H] myo-inositol in the total phosphoinositides in lungs and an increase in total inositol phosphate pool. There was a significant reduction in the contents and turnover rate of phosphatidyl inositol and phosphatidyl inositol monophosphate. Supplementation of diet with L-lysine and DL-Threonine had a reversing effect on total pool of phosphoinositides and, the metabolism of phosphatidyl inositol bisphosphate and phosphatidyl inositol. In phosphatidyl choline metabolism, the dietary protein deficiency led to a decrease in incorporation of [14C-methyl] choline-chloride in total phospholipids. In contrast, its incorporation increased in phosphatidyl choline pool. The contents of phosphatidyl choline and residue, incorporation of [14C-methyl] choline-chloride in them and their turnover rate also increased. Supplementation of diet had a reversal effect on most of these parameters. Phosphorylation of proteins of 84, 47, 35 and 16 kDa was identified to be mediated by PKC. In dietary protein deficiency, phosphorylation of all these proteins, except that of 47 kDa, increased. Supplementation of diet reversed the pattern except that of 84 kDa. The findings suggest that changes in phospholipid metabolism in dietary protein deficiency may effect the activity of PKC thereby influencing the phosphorylation of its substrate proteins and hence associated functions that may lead to pathophysiology of lung.
Spontaneous resolution of binary copper(II) complexes with racemic dipeptides: crystal structures of glycyl-L-alpha-amino-n-butyrato copper(II) monohydrate, glycyl-D-valinato copper(II) hemihydrate, and glycyl-L-valinato copper(II) hemihydrate.[Pubmed:15963569]
J Inorg Biochem. 2005 Aug;99(8):1611-8.
Copper(II) complexes with glycyl-DL-alpha-amino-n-butyric acid (H2gly-DL-but), glycyl-DL-valine (H2gly-DL-val), glycyl-DL-norleucine (H2gly-DL-norleu), glycyl-DL-Threonine (H2gly-DL-thr), glycyl-DL-serine (H2gly-DL-ser), glycyl-DL-phenylalanine (H2gly-DL-phe), and glycyl-L-valine (H2gly-L-val), have been prepared and characterized by IR, powder diffuse reflection, CD and ORD spectra, and magnetic susceptibility measurements, and by single-crystal X-ray diffraction. The crystal structures of the copper complex with H2gly-DL-but, the copper complex with H2gly-DL-val, and [Cu(gly-L-val)]n.0.5nH2O have been determined by a single-crystal X-ray diffraction method. As for the structure of the copper complex with H2gly-DL-but, the configuration around the asymmetric carbon atom is similar to that of [Cu(gly-L-val)]n.0.5nH2O. Therefore it is concluded that the copper complex with H2gly-DL-but is [Cu(gly-L-but)]n.nH2O. On the contrary, as for the structure of the copper complex with H2gly-DL-val, the configuration around the asymmetric carbon atom is different from that of [Cu(gly-L-val)]n.0.5nH2O. Therefore it is concluded that the copper complex with H2gly-dl-val is [Cu(gly-D-val)]n.0.5nH2O. So during the crystallization of the copper(II) complexes with H2gly-DL-but and H2gly-DL-val, spontaneous resolution has been observed; the four complexes have separated as [Cu(gly-D-but)]n.nH2O, [Cu(gly-L-but)]n.nH2O, [Cu(gly-D-val)]n.0.5nH2O, and [Cu(gly-L-val)]n.0.5nH2O, respectively. [Cu(gly-L-but)]n.nH2O is orthorhombic with the space group P2(1)2(1)2(1). [Cu(gly-D-val)]n.0.5nH2O and [Cu(gly-L-val)]n.0.5nH2O are monoclinic with the space group C2. In these complexes, the copper atom is in a square-pyramidal geometry, ligated by a peptide nitrogen atom, an amino nitrogen atom, a carboxyl oxygen atom, and a carboxyl oxygen atom and a peptide oxygen atom from neighboring molecules. So these complexes consist of a two-dimensional polymer chain bridged by a carboxyl oxygen atom and a peptide oxygen atom from neighboring molecules. The axial oxygen atom is located above the basal plane and the side chain of an amino acid is located below it. These polymer chains consist of only one or the other type of optical isomers; no racemic dipeptides are found. Therefore, spontaneous resolution has been observed in the crystallization of copper(II) complexes with H2gly-DL-but and H2gly-DL-val. The crystal structure of [Cu(gly-D-val)]n.0.5nH2O agrees almost completely with that of [Cu(gly-L-val)]n.0.5nH2O, except for the configuration around the asymmetric carbon atom.


