3-HydroxybenzaldehydeCAS# 100-83-4 |
Quality Control & MSDS
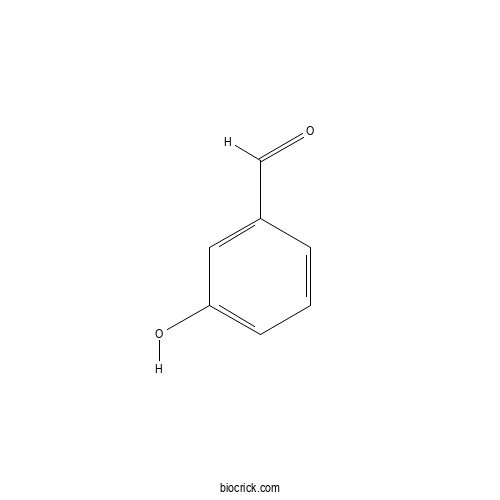
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100-83-4 | SDF | Download SDF |
| PubChem ID | 101 | Appearance | Powder |
| Formula | C7H6O2 | M.Wt | 122.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxybenzaldehyde | ||
| SMILES | C1=CC(=CC(=C1)O)C=O | ||
| Standard InChIKey | IAVREABSGIHHMO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6O2/c8-5-6-2-1-3-7(9)4-6/h1-5,9H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3-Hydroxybenzaldehyde, a novel therapeutic agent, has shown vasculoprotective potency in both in vitro and in vivo. 3-Hydroxybenzaldehyde has antioxidant and antibacterial activityies. | |||||

3-Hydroxybenzaldehyde Dilution Calculator

3-Hydroxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.19 mL | 40.95 mL | 81.9001 mL | 163.8002 mL | 204.7502 mL |
| 5 mM | 1.638 mL | 8.19 mL | 16.38 mL | 32.76 mL | 40.95 mL |
| 10 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 50 mM | 0.1638 mL | 0.819 mL | 1.638 mL | 3.276 mL | 4.095 mL |
| 100 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Heptacosanol
Catalog No.:BCN0107
CAS No.:2004-39-9
- Physalien
Catalog No.:BCN0106
CAS No.:144-67-2
- Rigosertib (ON-01910)
Catalog No.:BCN0105
CAS No.:1225497-78-8
- N-Malonyl DL-tryptophan
Catalog No.:BCN0104
CAS No.:3184-74-5
- trans-2-Hexen-1-ol
Catalog No.:BCN0103
CAS No.:928-95-0
- trans-2-Hexen-1-al
Catalog No.:BCN0102
CAS No.:6728-26-3
- 3,6-Dihydroxyflavone
Catalog No.:BCN0101
CAS No.:108238-41-1
- Heptyl acetate
Catalog No.:BCN0100
CAS No.:112-06-1
- Sieboldin
Catalog No.:BCN0099
CAS No.:18777-73-6
- (-)-Dihydrocarvyl acetate
Catalog No.:BCN0098
CAS No.:20777-49-5
- Piperitone
Catalog No.:BCN0097
CAS No.:89-81-6
- 1,4-Anthraquinone
Catalog No.:BCN0096
CAS No.:635-12-1
- DL-Threonine
Catalog No.:BCN0109
CAS No.:80-68-2
- Furfuryl acetate
Catalog No.:BCN0110
CAS No.:623-17-6
- Eupatorin-5-methylether
Catalog No.:BCN0111
CAS No.:21764-09-0
- Dehydroascorbic acid
Catalog No.:BCN0112
CAS No.:490-83-5
- alpha-Pinene oxide
Catalog No.:BCN0113
CAS No.:1686-14-2
- (+)-Isocorydine hydrochloride
Catalog No.:BCN0114
CAS No.:13552-72-2
- Sempervirine nitrate
Catalog No.:BCN0115
CAS No.:17994-15-9
- (-)-Carveol
Catalog No.:BCN0116
CAS No.:99-48-9
- Tricetinidin chloride
Catalog No.:BCN0117
CAS No.:65618-21-5
- Lactupicrin
Catalog No.:BCN0118
CAS No.:65725-11-3
- VX-702
Catalog No.:BCN0119
CAS No.:479543-46-9
- (+)-Longifolene
Catalog No.:BCN0120
CAS No.:475-20-7
Formal enantioselective synthesis of nhatrangin A.[Pubmed:32101216]
Org Biomol Chem. 2020 Mar 11;18(10):1949-1956.
A new and straightforward synthesis of the C1-C7 core fragment of nhatrangin A was achieved in 14 steps from achiral 3-Hydroxybenzaldehyde, without the need of chiral reagents or enzymatic resolution to introduce the chiral centers. The key asymmetric steps include in particular a highly enantioselective organocatalyzed Michael addition on an aryl vinyl ketone, a Sharpless asymmetric epoxidation and a subsequent regioselective ring opening of the resulting chiral epoxide. This work represents the first formal enantioselective synthesis of nhatrangin A.
Degradation kinetics and mechanism of 3-Chlorobenzoic acid in anoxic water environment using graphene/TiO2 as photocatalyst.[Pubmed:30526405]
Environ Technol. 2020 Jul;41(17):2165-2179.
Degradation kinetics and mechanism of 3-Chlorobenzoic acid (3-CBA) in anoxic water environment using graphene/TiO2 (GR/TiO2) as photocatalyst had been investigated. The effects of various parameters such as catalyst dosage, pH, initial concentration, catalyst reuse and dissolved oxygen (DO) on 3-CBA photocatalytic degradation kinetics were studied. The qualitative and quantitative analysis for degradation intermediate products and parent compound were studied by using HPLC, HPLC/MS/MS and IC technologies. The results show that the residual concentration of 3-CBA has a good linear relationship and its correlation coefficient R (2) are all greater than 0.985 by Langmuir-Hinshelwood (L-H) dynamic model under different photocatalytic degradation conditions. Some oxidative degradation products such as 3-chlorophenol, resorcinol, and hydroxyquinol are generated, and some reductive degradation products such as 3-chlorobenzaldehyde, 3-Hydroxybenzaldehyde, 3-hydroxybenzyl alcohol, and cyclohexanediol are produced, and part of 3-CBA are mineralized to generate CO2 when DO is in the range of 0.5-1.0 mg/L; When DO is less than 0.28 mg/L, photocatalytic reduction mainly occurs. The results provide a theoretical basis for photocatalytic in situ remediation of pollutants in anoxic water environment.
Enzymatic and Chemoenzymatic Three-Step Cascades for the Synthesis of Stereochemically Complementary Trisubstituted Tetrahydroisoquinolines.[Pubmed:28727894]
Angew Chem Int Ed Engl. 2017 Oct 2;56(41):12503-12507.
Chemoenzymatic and enzymatic cascade reactions enable the synthesis of complex stereocomplementary 1,3,4-trisubstituted tetrahydroisoquinolines (THIQs) with three chiral centers in a step-efficient and selective manner without intermediate purification. The cascade employs inexpensive substrates (3-Hydroxybenzaldehyde and pyruvate), and involves a carboligation step, a subsequent transamination, and finally a Pictet-Spengler reaction with a carbonyl cosubstrate. Appropriate selection of the carboligase and transaminase enzymes enabled the biocatalytic formation of (1R,2S)-metaraminol. Subsequent cyclization catalyzed either enzymatically by a norcoclaurine synthase or chemically by phosphate resulted in opposite stereoselectivities in the products at the C1 position, thus providing access to both orientations of the THIQ C1 substituent. This highlights the importance of selecting from both chemo- and biocatalysts for optimal results.
Effect of Various Compounds Blocking the Colony Pigmentation on the Aflatoxin B1 Production by Aspergillus flavus.[Pubmed:27801823]
Toxins (Basel). 2016 Oct 28;8(11). pii: toxins8110313.
Aflatoxins and melanins are the products of a polyketide biosynthesis. In this study, the search of potential inhibitors of the aflatoxin B1 (AFB1) biosynthesis was performed among compounds blocking the pigmentation in fungi. Four compounds-three natural (thymol, 3-Hydroxybenzaldehyde, compactin) and one synthetic (fluconazole)-were examined for their ability to block the pigmentation and AFB1 production in Aspergillus flavus. All compounds inhibited the mycelium pigmentation of a fungus growing on solid medium. At the same time, thymol, fluconazole, and 3-Hydroxybenzaldehyde stimulated AFB1 accumulation in culture broth of A. flavus under submerged fermentation, whereas the addition of 2.5 mug/mL of compactin resulted in a 50x reduction in AFB1 production. Moreover, compactin also suppressed the sporulation of A. flavus on solid medium. In vivo treatment of corn and wheat grain with compactin (50 mug/g of grain) reduced the level of AFB1 accumulation 14 and 15 times, respectively. Further prospects of the compactin study as potential AFB1 inhibitor are discussed.
Structural Analysis of an Evolved Transketolase Reveals Divergent Binding Modes.[Pubmed:27767080]
Sci Rep. 2016 Oct 21;6:35716.
The S385Y/D469T/R520Q variant of E. coli transketolase was evolved previously with three successive smart libraries, each guided by different structural, bioinformatical or computational methods. Substrate-walking progressively shifted the target acceptor substrate from phosphorylated aldehydes, towards a non-phosphorylated polar aldehyde, a non-polar aliphatic aldehyde, and finally a non-polar aromatic aldehyde. Kinetic evaluations on three benzaldehyde derivatives, suggested that their active-site binding was differentially sensitive to the S385Y mutation. Docking into mutants generated in silico from the wild-type crystal structure was not wholly satisfactory, as errors accumulated with successive mutations, and hampered further smart-library designs. Here we report the crystal structure of the S385Y/D469T/R520Q variant, and molecular docking of three substrates. This now supports our original hypothesis that directed-evolution had generated an evolutionary intermediate with divergent binding modes for the three aromatic aldehydes tested. The new active site contained two binding pockets supporting pi-pi stacking interactions, sterically separated by the D469T mutation. While 3-formylbenzoic acid (3-FBA) preferred one pocket, and 4-FBA the other, the less well-accepted substrate 3-Hydroxybenzaldehyde (3-HBA) was caught in limbo with equal preference for the two pockets. This work highlights the value of obtaining crystal structures of evolved enzyme variants, for continued and reliable use of smart library strategies.
Vasculoprotective Effects of 3-Hydroxybenzaldehyde against VSMCs Proliferation and ECs Inflammation.[Pubmed:27002821]
PLoS One. 2016 Mar 22;11(3):e0149394.
3-Hydroxybenzaldehyde (3-HBA) is a precursor compound for phenolic compounds like Protocatechuic aldehyde (PCA). From recent reports, PCA has shown vasculoprotective potency, but the effects of 3-HBA remain unclear. The aim of this study is to investigate the vasculoprotective effects of 3-HBA in endothelial cells, vascular smooth muscle cells and various animal models. We tested effects of 3-HBA in both vitro and vivo. 3-HBA showed that it prevents PDGF-induced vascular smooth muscle cells (VSMCs) migration and proliferation from MTS, BrdU assays and inhibition of AKT phosphorylation. It arrested S and G0/G1 phase of VSMC cell cycle in PI staining and it also showed inhibited expression levels of Rb1 and CD1. In human umbilical vein endothelial cells (HUVECs), 3-HBA inhibited inflammatory markers and signaling molecules (VCAM-1, ICAM-1, p-NF-kappaB and p-p38). For ex vivo, 3-HBA has shown dramatic effects in suppressing the sprouting from aortic ring of Spargue Dawley (SD) rats. In vivo data supported the vasculoprotective effects of 3-HBA as it inhibited angiogenesis from Matrigel Plug assay in C57BL6 mouse, prevented ADP-induced thrombus generation, increased blood circulation after formation of thrombus, and attenuated neointima formation induced by common carotid artery balloon injury of SD rats. 3-HBA, a novel therapeutic agent, has shown vasculoprotective potency in both in vitro and in vivo.
A ratiometric fluorescent probe for rapid, sensitive and selective detection of sulfur dioxide with large Stokes shifts by single wavelength excitation.[Pubmed:26177153]
Org Biomol Chem. 2015 Aug 28;13(32):8663-8.
4-(Benzothiazol-2-yl)-3-Hydroxybenzaldehyde, probe 1, has been developed as a ratiometric fluorescent probe for the sensitive and selective detection of sulfur dioxide with a fast response time (within seconds). This probe itself exhibited a yellow emission (lambdamaxem = 563 nm) with a 186 nm Stokes shift. Upon treatment with sulfite anions, this probe instantaneously displayed a blue emission (lambdamaxem = 467 nm) with a 133 nm Stokes shift. The large Stokes shifts and absorption spectral overlap of probe 1 both in the absence and in the presence of SO3(2-)/HSO3(-) allow the fluorescence detection under single wavelength excitation. Importantly, this ratiometric probe was successfully demonstrated for the imaging of intracellular sulfite anions in living cells.
Total mineralization of sulfamethoxazole and aromatic pollutants through Fe2+-montmorillonite catalyzed ozonation.[Pubmed:26118641]
J Hazard Mater. 2015 Nov 15;298:338-50.
The catalytic activity and selectivity of montmorillonite exchanged with Na(+), Fe(2+), Co(2+), Ni(2+) and Cu(2+) cations were comparatively investigated in the ozonation of sulfamethoxazole (SMX). Chlorobenzene, benzoic acid, 4-nitrobenzoic acid, 3-Hydroxybenzaldehyde, 4-nitrophenol and phenol were used as probe molecules having structural similarity with SMX oxidation intermediates. UV-vis spectrophometry and chemical oxygen demand (COD) measurements showed that Fe(II)-Mt and, to a lesser extent, Co(II)-Mt produce total mineralization of all organic substrates in less than 40 min. Combined HPLC-mass spectrometry revealed a reverse proportionality between the degradation time and molecular size of the organic substrates. Oxalic acid was recognized as a common bottleneck in the ozonation of any organic substrates. Ozonation initially obeyed a first order kinetics, but adsorption took place after 3-5 min, inducing changes in the mechanisms pathways. These findings may be useful for tailoring optimum oxidative treatment of waters without accumulation of hazardous derivatives.
Second generation engineering of transketolase for polar aromatic aldehyde substrates.[Pubmed:25765309]
Enzyme Microb Technol. 2015 Apr;71:45-52.
Transketolase has significant industrial potential for the asymmetric synthesis of carboncarbon bonds with new chiral centres. Variants evolved on propanal were found previously with nascent activity on polar aromatic aldehydes 3-formylbenzoic acid (3-FBA), 4-formylbenzoic acid (4-FBA), and 3-Hydroxybenzaldehyde (3-HBA), suggesting a potential novel route to analogues of chloramphenicol. Here we evolved improved transketolase activities towards aromatic aldehydes, by saturation mutagenesis of two active-site residues (R358 and S385), predicted to interact with the aromatic substituents. S385 variants selectively controlled the aromatic substrate preference, with up to 13-fold enhanced activities, and KM values comparable to those of natural substrates with wild-type transketolase. S385E even completely removed the substrate inhibition for 3-FBA, observed in all previous variants. The mechanisms of catalytic improvement were both mutation type and substrate dependent. S385E improved 3-FBA activity via kcat, but reduced 4-FBA activity via KM. Conversely, S385Y/T improved 3-FBA activity via KM and 4-FBA activity via kcat. This suggested that both substrate proximity and active-site orientation are very sensitive to mutation. Comparison of all variant activities on each substrate indicated different binding modes for the three aromatic substrates, supported by computational docking. This highlights a potential divergence in the evolution of different substrate specificities, with implications for enzyme engineering.
Nontargeted GC-MS approach for volatile profile of toasting in cherry, chestnut, false acacia, and ash wood.[Pubmed:24809897]
J Mass Spectrom. 2014 May;49(5):353-70.
By using a nontargeted GC-MS approach, 153 individual volatile compounds were found in extracts from untoasted, light toasted and medium-toasted cherry, chestnut, false acacia, as well as European and American ash wood, used in cooperage for aging wines, spirits and other beverages. In all wood types, the toasting provoked a progressive increase in carbohydrate derivatives, lactones and lignin constituents, along with a variety of other components, thus increasing the quantitative differences among species with the toasting intensity. The qualitative differences in the volatile profiles allow for identifying woods from cherry (being p-anisylalcohol, p-anisylaldehyde, p-anisylacetone, methyl benzoate and benzyl salicylate detected only in this wood), chestnut (cis and trans whisky lactone) and false acacia (resorcinol, 3,4-dimethoxyphenol, 2,4-dihydroxy benzaldehyde, 2,4-dihydroxyacetophenone, 2,4-dihydroxypropiophenone and 2,4-dihydroxy-3-methoxyacetophenone), but not those from ash, because of the fact that all compounds present in this wood are detected in at least one other. However, the quantitative differences can be clearly used to identify toasted ash wood, with tyrosol being most prominent, but 2-furanmethanol, 3- and 4-ethylcyclotene, alpha-methylcrotonolactone, solerone, catechol, 3-methylcatechol and 3-Hydroxybenzaldehyde as well. Regarding oak wood, its qualitative volatile profile could be enough to distinguish it from cherry and acacia woods, and the quantitative differences from chestnut (vanillyl ethyl ether, isoacetovanillone, butirovanillone, 1-(5-methyl-2-furyl)-2-propanone and 4-hydroxy-5,6-dihydro-(2H)-pyran-2-one) and ash toasted woods.
Purification and characterization of NAD+ -dependent salicylaldehyde dehydrogenase from carbaryl-degrading Pseudomonas sp. strain C6.[Pubmed:24122667]
Appl Biochem Biotechnol. 2014 Jan;172(2):806-19.
NAD+-dependent salicylaldehyde dehydrogenase (SALDH) which catalyzes the oxidation of salicylaldehyde to salicylate was purified form carbaryl-degrading Pseudomonas sp. strain C6. The enzyme was found to be a functional homotrimer (150 kDa) with subunit molecular mass of 50 kDa and contained calcium (1.8 mol/mol of enzyme). These properties were found to be unique. External addition of metal ions showed no effect on the activity and addition of chelators showed moderate inhibition of the activity. Potassium ions were found to enhance the activity significantly. SALDH showed higher affinity for salicylaldehyde (Km = 4.5 muM) and accepts mono- as well as di-aromatic aldehydes; however it showed poor activity on aliphatic aldehydes. Chloro-/nitro-substituted benzaldehydes were potent substrate inhibitors as compared to benzaldehyde and 3-Hydroxybenzaldehyde, while 2-naphthaldehyde and salicylaldehyde were moderate. The kinetic data revealed that SALDH, though having broad specificity, is more efficient for the oxidation of salicylaldehyde as compared to other aromatic aldehyde dehydrogenases which gives an advantage for Pseudomonas sp. strain C6 to bioremediate carbaryl and other aromatic aldehydes efficiently.
Quantum chemical studies, vibrational analysis, molecular structure, first order hyper polarizability, NBO and HOMO-LUMO analysis of 3-hydroxybenzaldehyde and its cation.[Pubmed:23892343]
Spectrochim Acta A Mol Biomol Spectrosc. 2013 Nov;115:789-99.
FT-IR spectroscopy has been applied to investigate the potential nonlinear optical (NLO) material 3-Hydroxybenzaldehyde (3HBA). The equilibrium geometry, Fukui function, harmonic vibrational frequencies, infrared intensities, and thermodynamic properties of 3HBA and its cation were calculated by HF/6-31G(d,p) and density functional theory B3LYP/6-31G(d,p), B3LYP/6-311++G(d,p) methods. The first order hyperpolarizability (betatotal) of this molecular system and related properties (beta, mu, and Deltaalpha) are calculated based on the finite-field approach. Stability of the molecule arising from hyperconjugative interactions, charge delocalization and intramolecular hydrogen bond-like weak interaction has been analyzed using natural bond orbital (NBO) analysis by using B3LYP/6-311++G(d,p) method. The results show that electron density (ED) in the sigma(*) and pi(*)anti-bonding orbitals and second-order delocalization energies E((2)) confirm the occurrence of intramolecular charge transfer (ICT) within the molecule. The thermal stability of 3HBA and its cation is studied by the thermogravimetric analysis (TGA). The harmonic-vibrational frequencies were calculated and the scaled values have been compared with experimental FT-IR spectrum. The observed and the calculated frequencies are found to be in good agreement. The experimental spectrum also coincides satisfactorily with those of theoretically constructed spectrograms.
The first synthesis of [11C]J147, a new potential PET agent for imaging of Alzheimer's disease.[Pubmed:23237833]
Bioorg Med Chem Lett. 2013 Jan 15;23(2):524-7.
J147 was synthesized from 2,4-dimethylphenylhydrazine hydrochloride and 3-methoxybenzaldehyde in 2 steps with 71% overall yield. The precursor desmethyl-J147 was synthesized from 3-Hydroxybenzaldehyde and 2,4-dimethylphenylhydrazine hydrochloride in 4 steps with 63% overall yield. [(11)C]J147 was prepared from desmethyl-J147 with [(11)C]CH(3)OTf through O-[(11)C]methylation and isolated by HPLC combined with solid-phase extraction (SPE) in 35-50% radiochemical yield based on [(11)C]CO(2) and decay corrected to end of bombardment (EOB), with 370-740 GBq/mumol specific activity at EOB.
New 1,3,4-bisthiadiazolines: synthesis, characterization and antimicrobial evaluations.[Pubmed:22832010]
Spectrochim Acta A Mol Biomol Spectrosc. 2012 Nov;97:470-8.
The bisthiadiazolines 4a-4g have been synthesized in good yields from the cyclization reactions of bisthiosemicarbazones 3a-3g with acetic anhydride. The condensation reaction of dibenzaldehydes 2a-2g with thiosemicarbazide in alcoholic medium provided 3a-3g and former were obtained from the O-alkylation of 3-Hydroxybenzaldehyde with suitable 1,omega-dibromoalkanes under alkaline conditions in the presence of dry EtOH/DMF. The intermediates 3a-3g and bishetrocyclics 4a-4g were also screened for their in vitro antimicrobial activities against seven bacterial strains (Klubsellia pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Straphylococcus aureus, Bacillius subtilis, Pseudomonas fluorescens and Streptoccus pyrogens) and five fungi strains (Aspergillius janus, Pencillium glabrum, Fusarium oxysporum, Aspergillus sclerotiorum, Aspergillus niger). The compounds 3f, 3g, 4f &4g were found to be significantly active against the tested microorganisms.


