2,4,6-Trihydroxybenzoic acidCAS# 83-30-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83-30-7 | SDF | Download SDF |
| PubChem ID | 66520 | Appearance | Powder |
| Formula | C7H6O5 | M.Wt | 170.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,4,6-trihydroxybenzoic acid | ||
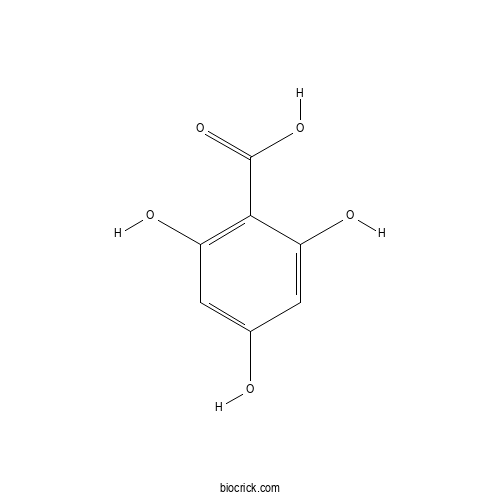
| SMILES | C1=C(C=C(C(=C1O)C(=O)O)O)O | ||
| Standard InChIKey | IBHWREHFNDMRPR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6O5/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2,8-10H,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2,4,6-Trihydroxybenzoic acid may mediate its effects through a CDK- and SLC5A8-dependent pathway contributing to the prevention of colorectal cancer. | |||||

2,4,6-Trihydroxybenzoic acid Dilution Calculator

2,4,6-Trihydroxybenzoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8789 mL | 29.3945 mL | 58.7889 mL | 117.5779 mL | 146.9724 mL |
| 5 mM | 1.1758 mL | 5.8789 mL | 11.7578 mL | 23.5156 mL | 29.3945 mL |
| 10 mM | 0.5879 mL | 2.9394 mL | 5.8789 mL | 11.7578 mL | 14.6972 mL |
| 50 mM | 0.1176 mL | 0.5879 mL | 1.1758 mL | 2.3516 mL | 2.9394 mL |
| 100 mM | 0.0588 mL | 0.2939 mL | 0.5879 mL | 1.1758 mL | 1.4697 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetin 3,5,7,3,4-pentamethyl ether
Catalog No.:BCN0087
CAS No.:1247-97-8
- (-)-Myrtenol
Catalog No.:BCN0086
CAS No.:19894-97-4
- Phenethyl alcohol
Catalog No.:BCN0085
CAS No.:60-12-8
- Cascaroside A
Catalog No.:BCN0084
CAS No.:53823-08-8
- 3-Hydroxy-6-methoxyflavone
Catalog No.:BCN0083
CAS No.:93176-00-2
- Evernic acid
Catalog No.:BCN0082
CAS No.:537-09-7
- Sabinyl acetate
Catalog No.:BCN0081
CAS No.:53833-85-5
- 3,5-Dihydroxy-4-methylbenzoic acid
Catalog No.:BCN0080
CAS No.:28026-96-2
- 3,5,7-Trihydroxy-3',4',5'-trimethoxyflavone
Catalog No.:BCN0079
CAS No.:146132-95-8
- 2-Octanone
Catalog No.:BCN0078
CAS No.:111-13-7
- Cimiaceroside A
Catalog No.:BCN0077
CAS No.:210643-83-7
- 3,4-Dihydroxy-5-methoxybenzoic acid
Catalog No.:BCN0076
CAS No.:3934-84-7
- Serpentine hydrogen tartrate
Catalog No.:BCN0089
CAS No.:58782-36-8
- Fumarprotocetraric acid
Catalog No.:BCN0090
CAS No.:489-50-9
- N-Formylcytisine
Catalog No.:BCN0091
CAS No.:53007-06-0
- (-)-Perillyl alcohol
Catalog No.:BCN0092
CAS No.:18457-55-1
- 2-Methoxy-1,4-naphthoquinone
Catalog No.:BCN0093
CAS No.:2348-82-5
- Neoarctin B
Catalog No.:BCN0094
CAS No.:155969-67-8
- Rosmaquinone
Catalog No.:BCN0095
CAS No.:121927-71-7
- 1,4-Anthraquinone
Catalog No.:BCN0096
CAS No.:635-12-1
- Piperitone
Catalog No.:BCN0097
CAS No.:89-81-6
- (-)-Dihydrocarvyl acetate
Catalog No.:BCN0098
CAS No.:20777-49-5
- Sieboldin
Catalog No.:BCN0099
CAS No.:18777-73-6
- Heptyl acetate
Catalog No.:BCN0100
CAS No.:112-06-1
Qualitative and quantitative analysis of phenolic acid glycosides in Ginkgo biloba L. leaf, G. biloba leaf extract and its injection.[Pubmed:32790185]
Biomed Chromatogr. 2020 Dec;34(12):e4964.
Ginkgo biloba L. leaf (GBL) is one of the most commonly used medicinal plants in the world. Phenolic acids with biological activities have a relatively high content in G. biloba leaf extracts (GBE); therefore they are of great significance for the quality control of GBL, GBE and its preparations. However, there have been few studies focused on their analysis. In this work, 12 phenolic acids, including 11 phenolic acid glycosides, were identified by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC-Q-TOF/MS). Then, a method combining enzymolysis with HPLC was established for quantification of phenolic acid glycosides. It was found that the aglycones of phenolic acid glycosides mainly comprised five phenolic acids: 2,4,6-Trihydroxybenzoic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid and p-coumaric acid. The quantitative method was validated, and the correlation coefficient (0.9993-0.9999), recovery (>/=88.4%), repeatability (
Do Aspirin and Flavonoids Prevent Cancer through a Common Mechanism Involving Hydroxybenzoic Acids?-The Metabolite Hypothesis.[Pubmed:32397626]
Molecules. 2020 May 10;25(9). pii: molecules25092243.
Despite decades of research to elucidate the cancer preventive mechanisms of aspirin and flavonoids, a consensus has not been reached on their specific modes of action. This inability to accurately pinpoint the mechanism involved is due to the failure to differentiate the primary targets from its associated downstream responses. This review is written in the context of the recent findings on the potential pathways involved in the prevention of colorectal cancers (CRC) by aspirin and flavonoids. Recent reports have demonstrated that the aspirin metabolites 2,3-dihydroxybenzoic acid (2,3-DHBA), 2,5-dihydroxybenzoic acid (2,5-DHBA) and the flavonoid metabolites 2,4,6-Trihydroxybenzoic acid (2,4,6-THBA), 3,4-dihydroxybenzoic acid (3,4-DHBA) and 3,4,5-trihydroxybenzoic acid (3,4,5-THBA) were effective in inhibiting cancer cell growth in vitro. Limited in vivo studies also provide evidence that some of these hydroxybenzoic acids (HBAs) inhibit tumor growth in animal models. This raises the possibility that a common pathway involving HBAs may be responsible for the observed cancer preventive actions of aspirin and flavonoids. Since substantial amounts of aspirin and flavonoids are left unabsorbed in the intestinal lumen upon oral consumption, they may be subjected to degradation by the host and bacterial enzymes, generating simpler phenolic acids contributing to the prevention of CRC. Interestingly, these HBAs are also abundantly present in fruits and vegetables. Therefore, we suggest that the HBAs produced through microbial degradation of aspirin and flavonoids or those consumed through the diet may be common mediators of CRC prevention.
2,4,6-Trihydroxybenzoic Acid-Catalyzed Oxidative Ugi Reactions with Molecular Oxygen via Homo- and Cross-Coupling of Amines.[Pubmed:31432673]
J Org Chem. 2019 Sep 20;84(18):11562-11571.
Metal-free, oxidative four-component Ugi reactions (U-4CRs) were conducted to synthesize dipeptides from two different amines, isocyanides, and carboxylic acids using 2,4,6-Trihydroxybenzoic acid catalyst in O2 atmosphere. The organocatalytic U-4CRs proceed via oxidative cross-coupling of benzylamines with other aliphatic or aromatic amines to form imines, followed by condensation with isocyanides and carboxylic acids. The U-4CRs via cross-coupling of amines are rare, and the simple, metal-free procedures are advantageous for further applications in drug and heterocycle syntheses.
Structural insights into anhydrous and monohydrated forms of 2,4,6-trihydroxybenzoic acid based on Raman and terahertz spectroscopic characterization.[Pubmed:31394390]
Spectrochim Acta A Mol Biomol Spectrosc. 2020 Jan 5;224:117436.
In order to characterize molecular structures of 2,4,6-Trihydroxybenzoic acid (PCA) by means of vibrational spectroscopic techniques, we report investigation of PCA monohydrated form and its anhydrous polymorphic one by using terahertz and Raman spectral characterization. The experimental THz spectra show that the monohydrated PCA only has two absorption bands at 0.69 and 1.65 THz respectively in the frequency region from 0.2 to 1.8 THz, meanwhile the anhydrous form has a few significantly different absorption bands at 0.75, 1.01, 1.46 and 1.64 THz, respectively. Furthermore, Raman spectra characterized such differences of vibrational modes shown within 200-1800cm(-1) region about the monohydrated and anhydrous forms of PCA. In view of various possible theoretical structural forms that may exist in anhydrous PCA and its monohydrated one, density functional theory calculations were performed to simulate optimized structures and vibrational mode of above two PCA polymorphic forms. Theoretical results and experimental THz/Raman spectra of anhydrous PCA show that the dimer synthon via the carboxylic group *** carboxyl group and its ortho-phenolic hydroxyl group inter-molecular hydrogen bonding interaction establishing the theoretical form I (AH-I) is more consistent with experimental observation than other theoretical forms (AH-II and AH-III). Meanwhile, the theoretical monohydrated form I (MH-I), which is formed by the linkage of carboxyl group and its ortho-phenolic hydroxyl group with water molecule, is also much more agreement with experimental spectral observations of PCA monohydrate than other monohydrated forms (MH-II and MH-III). Our study demonstrates effectively qualitative analysis of both micro-molecular structures and dehydrated transitions between anhydrous and hydrated polymorphic forms of PCA, thus providing rich information on the corresponding structural changes of anhydrous and hydrated PCAs due to various inter-molecular and intra-molecular interactions based on their finger-print vibrational spectra combined with theoretical simulations.
The Flavonoid Metabolite 2,4,6-Trihydroxybenzoic Acid Is a CDK Inhibitor and an Anti-Proliferative Agent: A Potential Role in Cancer Prevention.[Pubmed:30917530]
Cancers (Basel). 2019 Mar 26;11(3). pii: cancers11030427.
Flavonoids have emerged as promising compounds capable of preventing colorectal cancer (CRC) due to their anti-oxidant and anti-inflammatory properties. It is hypothesized that the metabolites of flavonoids are primarily responsible for the observed anti-cancer effects owing to the unstable nature of the parent compounds and their degradation by colonic microflora. In this study, we investigated the ability of one metabolite, 2,4,6-Trihydroxybenzoic acid (2,4,6-THBA) to inhibit Cyclin Dependent Kinase (CDK) activity and cancer cell proliferation. Using in vitro kinase assays, we demonstrated that 2,4,6-THBA dose-dependently inhibited CDKs 1, 2 and 4 and in silico studies identified key amino acids involved in these interactions. Interestingly, no significant CDK inhibition was observed with the structurally related compounds 3,4,5-trihydroxybenzoic acid (3,4,5-THBA) and phloroglucinol, suggesting that orientation of the functional groups and specific amino acid interactions may play a role in inhibition. We showed that cellular uptake of 2,4,6-THBA required the expression of functional SLC5A8, a monocarboxylic acid transporter. Consistent with this, in cells expressing functional SLC5A8, 2,4,6-THBA induced CDK inhibitory proteins p21(Cip1) and p27(Kip1) and inhibited cell proliferation. These findings, for the first time, suggest that the flavonoid metabolite 2,4,6-THBA may mediate its effects through a CDK- and SLC5A8-dependent pathway contributing to the prevention of CRC.
Salicylic acid metabolites and derivatives inhibit CDK activity: Novel insights into aspirin's chemopreventive effects against colorectal cancer.[Pubmed:29075787]
Int J Oncol. 2017 Dec;51(6):1661-1673.
Aspirin's potential as a drug continues to be evaluated for the prevention of colorectal cancer (CRC). Although multiple targets for aspirin and its metabolite, salicylic acid, have been identified, no unifying mechanism has been proposed to clearly explain its chemopreventive effects. Our goal here was to investigate the ability of salicylic acid metabolites, known to be generated through cytochrome P450 (CYP450) enzymes, and its derivatives as cyclin dependent kinase (CDK) inhibitors to gain new insights into aspirin's chemopreventive actions. Using in vitro kinase assays, for the first time, we demonstrate that salicylic acid metabolites, 2,3-dihydroxybenzoic acid (2,3-DHBA) and 2,5-dihydroxybenzoic acid (2,5-DHBA), as well as derivatives 2,4-dihydroxybenzoic acid (2,4-DHBA), 2,6-dihydroxybenzoic acid (2,6-DHBA), inhibited CDK1 enzyme activity. 2,3-DHBA and 2,6-DHBA did not inhibit CDK2 and 4; however, both inhibited CDK-6 activity. Interestingly, another derivative, 2,4,6-Trihydroxybenzoic acid (2,4,6-THBA) was highly effective in inhibiting CDK1, 2, 4 and 6 activity. Molecular docking studies showed that these compounds potentially interact with CDK1. Immunoblotting experiments showed that aspirin acetylated CDK1, and pre-incubation with salicylic acid and its derivatives prevented aspirin-mediated CDK1 acetylation, which supported the data obtained from molecular docking studies. We suggest that intracellularly generated salicylic acid metabolites through CYP450 enzymes within the colonic epithelial cells, or the salicylic acid metabolites generated by gut microflora may significantly contribute to the preferential chemopreventive effect of aspirin against CRC through inhibition of CDKs. This novel hypothesis and mechanism of action in aspirin's chemopreventive effects opens a new area for future research. In addition, structural modification to salicylic acid derivatives may prove useful in the development of novel CDK inhibitors in cancer prevention and treatment.
Biotransformation of quercetin by Gliocladium deliquescens NRRL 1086.[Pubmed:28939024]
Chin J Nat Med. 2017 Aug;15(8):615-624.
With an attempt to synthesize high-value isoquercitrin (quercetin-3-O-beta-D-glucopyranoside), we carried out the biotransformation of quercetin (1) by Gliocladium deliquescens NRRL 1086. Along with the aimed product quercetin 3-O-beta-D-glycoside (2), three additional metabolites, 2-protocatechuoyl-phlorogucinol carboxylic acid (3), 2,4,6-Trihydroxybenzoic acid (4), and protocatechuic acid (5), were also isolated. The time-course experiments revealed that there were two metabolic routes, regio-selectivity glycosylation and quercetin 2,3-dioxygenation, co-existing in the culture. Both glycosylation and oxidative cleavage rapidly took place after quercetin feeding; about 98% quercetin were consumed within the initial 8 h and the oxdized product (2-protocatechuoyl-phlorogucinol carboxylic acid) was hydrolyzed into two phenolic compounds (2,4,6-Trihydroxybenzoic acid and protocatechuic acid). We also investigated the impact of glucose content and metal ions on the two reactions and found that high concentrations of glucose significantly inhibited the oxidative cleavage and improved the yield of isoquercitrin and that Ca(2+), Fe(2+), Mn(2+), Mg(2+), and Zn(2+) inhibited glycosylation. To test the promiscuity of this culture, we selected other four flavonols as substrates; the results demonstrated its high regio-selectivity glycosylation ability towards flavonols at C-3 hydroxyl. In conclusion, our findings indicated that the versatile microbe of G. deliquescens NRRL 1086 maitained abundant enzymes, deserving further research.
The effect of Hg(II) ions on the free radicals of humic substances and their model compounds.[Pubmed:23411090]
Chemosphere. 2013 Jul;92(4):445-50.
Humic acids (HAs) and humin (HU) complexes with Hg(II) ions were studied using EPR, FTIR and CP MAS (13)C NMR spectroscopies. The analysis of the EPR spectra showed, especially for HA, a significant decrease in the g-factor value for the resulting Hg(II) radical species, as compared with that observed for the parent radicals. The concentration of the formed radical complexes was very low, precluding their detailed characterization. For this reason hydroxybenzenes and their benzoic acids were used as models of the humic substances' radical centres able to form radical ligands towards Hg(II) ions. The resulting radicals, characterized by a g-factor lower than that of the free electron (2.0006-2.00017), were efficiently produced upon Hg(II) complexation only for the hydroxybenzenes with their OH groups in the ortho position and, what is more important, the presence of carboxylic substituents was not necessary. It is most likely that formation of the radicals characterised by low g-factor proceeded in two steps. The first is oxidation process of the hydroxybenzenes and their derivatives resulting in a typical phenolic/semiquinone type radical while in the second step the radical is complexed by the excess of Hg(II), as the result spin density of the radical is shifted towards aromatic ring leading to the observed lowering of the g parameter value. The redox character of the process starting from Hg(II) was confirmed by the formation of elemental mercury. The g-factor of the semiquinone radical obtained from 2,4,6-Trihydroxybenzoic acid was not changed by Hg(II) ions coordination, as indicated by g =2.0034.
The phenolic acids from bacterial degradation of the mangiferin aglycone are quantified in the feces of pigs after oral ingestion of an extract of Cyclopia genistoides (honeybush tea).[Pubmed:20579527]
Nutr Res. 2010 May;30(5):348-57.
Polyphenols are cleaved by bacterial enzymes to form phenolic acid metabolites in the colon, where they may exert physiologic effects. For norathyriol, the aglycone of mangiferin, one of the major phenolic compounds present in Cyclopia genistoides (honeybush), a further bacterial degradation is likely; but knowledge of the importance of this metabolic process is very limited. Based on a hypothesized cleavage of the middle ring of norathyriol, this study was designed to determine phenolic cleavage products in the feces of pigs fed an extract of C genistoides. Pigs received 74 mg mangiferin per kilogram of body weight daily for 11 days; feces fractions were collected on day 11 and on the first 2 days after the last intake of extract. Several phenolic acids were detected in the feces samples, including 3-hydroxyphenylacetic acid; 4-hydroxybenzoic acid; 3,4-dihydroxybenzoic acid; 3,4-dihydroxyphenylacetic acid; 2,4,6-Trihydroxybenzoic acid; 3,4,5-trihydroxybenzoic acid (gallic acid); and phloroglucinol. However, in vivo formation was likely only for 3,4-dihydroxybenzoic acid; 3,4-dihydroxyphenylacetic acid; 2,4,6-Trihydroxybenzoic acid; and 3,4,5-trihydroxybenzoic acid because these were not present in the blank feces, in the animals' normal diet, or in the C genistoides extract. The fact that these amounts were very low suggests further degradation of the metabolites by intestinal microflora or absorption of the cleavage products by the colon.
Spectral alteration and degradation of cyanidin-3-glucoside exposed to pulsed electric field.[Pubmed:20163112]
J Agric Food Chem. 2010 Mar 24;58(6):3524-31.
Anthocyanins are polyphenol antioxidants that have been shown to prevent many chronic diseases. The compounds are not stable, so they tend to be decolorized or degraded during processing and storage. In this study, the spectral characteristics alteration and degradation products of cyanidin-3-glucoside (Cyd-3-glc) exposed to pulsed electric field (PEF) were investigated, and the reaction kinetics was discussed. The intensity of the UV-vis spectra decreased noticeably upon PEF treatment without modification of the spectral pattern. Protocatechuic acid and 2,4,6-Trihydroxybenzoic acid were identified as degradation products of both PEF and thermally treated Cyd-3-glc, but cyanidin present in thermally treated Cyd-3-glc was absent in PEF-treated Cyd-3-glc, indicating that the first step of Cyd-3-glc degradation induced by PEF was not the hydrolysis of glycosidic bonds, which was different from that of thermal degradation. With increased electric field intensity or treatment time, the degradation of Cyd-3-glc and the formation of protocatechuic acid were enhanced; their kinetics (except 7 kV/cm for protocatechuic acid formation) were well fitted to a first-order reaction. Meanwhile, a good correlation was present between Cyd-3-glc degradation and protocatechuic acid formation.
Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity.[Pubmed:16365058]
J Nutr. 2006 Jan;136(1):52-7.
Dietary flavonoids are poorly absorbed from the gastrointestinal tract. Colonic bacteria convert flavonoids into smaller phenolic acids (PA), which can be absorbed into the circulation and may contribute to the chemopreventive activity of the parent compounds. The purpose of our study was to determine whether flavonoids from green and black tea (GT, BT), citrus fruit with rutin (CF+R) and soy (S) supplements exposed to the same conditions in a dynamic in vitro model of the colon (TIM-2) will form the same phenolic acid products of microbial metabolism. About 600 mg of flavonoids from GT, BT, CF+R and S extracts were infused at t = 0 and 12 h into the TIM-2. Samples from the lumen and dialysate were collected at t = 0,4,8,12,16,24 and 28h. The flavonoid and PA concentrations were measured by HPLC and GC-MS. GT, BT, and CF+R formed 3-methoxy-4-hydroxyphenylacetic acid (3M4HPAA), 4-hydroxyphenyl acetic acid (4HPAA), 3,4-dihydroxyphenylacetic acid (3,4DHPAA), and 3-(3-hydroxyphenyl) propionic acid (3,3HPPA). BT flavonoids were also metabolized to 2,4,6-Trihydroxybenzoic acid (2,4,6THBA) and CF+R flavonoids to 3-(4-hydroxy-3-methoxyphenyl) propionic acid (3,4H3MPPA), 3-hydroxyphenyl acetic acid (3HPAA) and a small amount of hippuric acid. After S infusion, we found 3M4HPAA and 4HPAA only. Among these phenolic acids, only 3,4DHPAA exhibited antiproliferative activity in prostate and colon cancer cells. 3,4DHPAA was significantly (P < 0.005) more inhibitory in colon cancer cells (HCT116) compared with an immortalized normal intestinal epithelial cell line (IEC6). In summary, fermentation by intestinal microbes of GT, BT, C+R, and S flavonoids resulted in the conversion to the same major phenolic acids.
Acute toxicity of benzoic acids to the crustacean Daphnia magna.[Pubmed:15722097]
Chemosphere. 2005 Apr;59(2):255-61.
The acute immobilization toxicity of benzoic acids substituted with hydroxyl and/or methoxyl groups on the aromatic ring was determined for the freshwater crustacean Daphnia magna under neutralized condition (initial pH: 7.45+/-0.05). Toxicity, expressed as EC50 value, varied depending largely on the number and position of phenolic hydroxyl groups. Especially, benzoic acids with ortho-substituted hydroxyl groups were more toxic than benzoic acids with meta- and/or para-substituted hydroxyl groups. Whereas the limited data indicated that methoxyl substitution had relatively small and variable effects on the toxicity. Of the tested compounds, 2,4,6-Trihydroxybenzoic acid showed the highest toxicity with the 48 h EC50 of 10 micromol l-1. This was 700 times as toxic as the parent benzoic acid (48 h EC50=7.0 mmol l-1) and about two orders of magnitude higher than those previously reported for monohalogenated benzoic acid derivatives in Daphnia. Within the subgroups based on the number of hydroxyl groups (N(OH)), the toxicity variations due to the position of hydroxyl groups appeared to be correlated with the logarithms of n-octanol/water partition coefficients (logPow). The toxicity of benzoic acids existing almost entirely as their ionized forms could be expressed as simple structure-toxicity relationships using these two descriptors (N(OH) and logPow).
[Studies on chemical constitutents from Penthorum chinense Pursh].[Pubmed:12525050]
Zhongguo Zhong Yao Za Zhi. 2001 Apr;26(4):260-2.
OBJECTIVE: To study the chemical constituents of Penthorum chinense. METHOD: Silica gel and macroporous resin were used as adsorbent for isolation and purification. The chemical structures were elucidated by spectral analysis and chemical methods. RESULT: Three compounds were isolated from the water-extract of P. chinense. The structures were identified as 5-hydroxy-flavanone-7-O-beta-D-glucoside (I), 2,4,6-Trihydroxybenzoic acid (II) and quercetin(III), respectively. CONCLUSION: Compounds I and II were isolated from this plant for the first time.
Degradation products of cyanidin glycosides from tart cherries and their bioactivities.[Pubmed:11600045]
J Agric Food Chem. 2001 Oct;49(10):4924-9.
The bioactive anthocyanins present in tart cherries, Prunus cerasus L. (Rosaceae) cv. Balaton, are cyanidin 3-glucosylrutinoside (1), cyanidin 3-rutinoside (2), and cyanidin 3-glucoside (3). Cyanidin (4) is the major anthocyanidin in tart cherries. In our continued evaluation of the in vivo and in vitro efficacy of these anthocyanins to prevent inflammation and colon cancer, we have added these compounds to McCoy's 5A medium in an effort to identify their degradation products during in vitro cell culture studies. This resulted in the isolation and characterization of protocatechuic acid (5), the predominant degradation product. In addition, 2,4-dihydroxybenzoic acid (6) and 2,4,6-Trihydroxybenzoic acid (7) were identified as degradation products. However, these degradation products were not quantified. Compounds 5-7 were also identified as degradation products when anthocyanins were subjected to varying pH and thermal conditions. In cyclooxygenase (COX)-I and -II enzyme inhibitory assays, compounds 5-7 did not show significant activities when compared to the NSAIDs Naproxen, Celebrex, and Vioxx, or Ibuprofen, at 50 microM concentrations. However, at a test concentration of 50 microM, the antioxidant activity of protocatechuic acid (5) was comparable to those of the commercial antioxidants tert-butylhydroquinone (TBHQ), butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA), and superior to that of vitamin E at 10 microM concentrations.


