Sabinyl acetateCAS# 53833-85-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

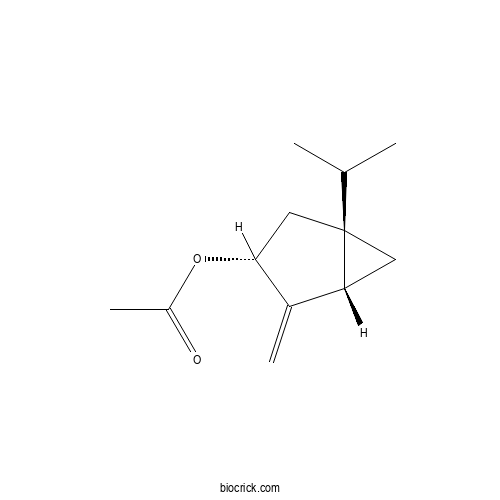
| Cas No. | 53833-85-5 | SDF | Download SDF |
| PubChem ID | 135251 | Appearance | Colourless-faint yellow oil |
| Formula | C12H18O2 | M.Wt | 194.3 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Acetic acid sabinyl ester | ||
| Solubility | Soluble in chloroform | ||
| Chemical Name | [(1S,3R,5S)-4-methylidene-1-propan-2-yl-3-bicyclo[3.1.0]hexanyl] acetate | ||
| SMILES | CC(C)C12CC1C(=C)C(C2)OC(=O)C | ||
| Standard InChIKey | PBWRFXQNNGSAQG-UTUOFQBUSA-N | ||
| Standard InChI | InChI=1S/C12H18O2/c1-7(2)12-5-10(12)8(3)11(6-12)14-9(4)13/h7,10-11H,3,5-6H2,1-2,4H3/t10-,11-,12+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sabinyl Acetate has antioxidant activity, it also shows antiimplantation effect on the abortifacient effect of Juniperus sabina essential oil. | |||||

Sabinyl acetate Dilution Calculator

Sabinyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1467 mL | 25.7334 mL | 51.4668 mL | 102.9336 mL | 128.667 mL |
| 5 mM | 1.0293 mL | 5.1467 mL | 10.2934 mL | 20.5867 mL | 25.7334 mL |
| 10 mM | 0.5147 mL | 2.5733 mL | 5.1467 mL | 10.2934 mL | 12.8667 mL |
| 50 mM | 0.1029 mL | 0.5147 mL | 1.0293 mL | 2.0587 mL | 2.5733 mL |
| 100 mM | 0.0515 mL | 0.2573 mL | 0.5147 mL | 1.0293 mL | 1.2867 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3,5-Dihydroxy-4-methylbenzoic acid
Catalog No.:BCN0080
CAS No.:28026-96-2
- 3,5,7-Trihydroxy-3',4',5'-trimethoxyflavone
Catalog No.:BCN0079
CAS No.:146132-95-8
- 2-Octanone
Catalog No.:BCN0078
CAS No.:111-13-7
- Cimiaceroside A
Catalog No.:BCN0077
CAS No.:210643-83-7
- 3,4-Dihydroxy-5-methoxybenzoic acid
Catalog No.:BCN0076
CAS No.:3934-84-7
- Isoarundinin II
Catalog No.:BCN0075
CAS No.:151538-56-6
- Gitoxin
Catalog No.:BCN0074
CAS No.:4562-36-1
- Procyanidin B4
Catalog No.:BCN0073
CAS No.:29106-51-2
- Undecanoic gamma-lactone
Catalog No.:BCN0072
CAS No.:104-67-6
- Homobutein
Catalog No.:BCN0071
CAS No.:34000-39-0
- Peucenidin
Catalog No.:BCN0070
CAS No.:33044-93-8
- Laricitrin
Catalog No.:BCN0069
CAS No.:53472-37-0
- Evernic acid
Catalog No.:BCN0082
CAS No.:537-09-7
- 3-Hydroxy-6-methoxyflavone
Catalog No.:BCN0083
CAS No.:93176-00-2
- Cascaroside A
Catalog No.:BCN0084
CAS No.:53823-08-8
- Phenethyl alcohol
Catalog No.:BCN0085
CAS No.:60-12-8
- (-)-Myrtenol
Catalog No.:BCN0086
CAS No.:19894-97-4
- Quercetin 3,5,7,3,4-pentamethyl ether
Catalog No.:BCN0087
CAS No.:1247-97-8
- 2,4,6-Trihydroxybenzoic acid
Catalog No.:BCN0088
CAS No.:83-30-7
- Serpentine hydrogen tartrate
Catalog No.:BCN0089
CAS No.:58782-36-8
- Fumarprotocetraric acid
Catalog No.:BCN0090
CAS No.:489-50-9
- N-Formylcytisine
Catalog No.:BCN0091
CAS No.:53007-06-0
- (-)-Perillyl alcohol
Catalog No.:BCN0092
CAS No.:18457-55-1
- 2-Methoxy-1,4-naphthoquinone
Catalog No.:BCN0093
CAS No.:2348-82-5
GC-MS Analysis of the Composition of the Essential Oil from Dendranthema indicum Var. Aromaticum Using Three Extraction Methods and Two Columns.[Pubmed:29510531]
Molecules. 2018 Mar 4;23(3). pii: molecules23030576.
Dendranthema indicum var. aromaticum, which is an aromatic plant with a strong and special fragrance throughout the whole plant, is used for the treatment of colds and headaches, and as a mosquito repellant in Shennongjia, Hubei province, China. To analyze the composition of the essential oil from this medicinal herb, we developed a gas chromatography-mass Spectrometry (GC-MS) method including microwave-assisted extraction, hydrodistillation and direct headspace analysis in two different stationary phase columns. In total, 115 volatile compounds were identified, of which 90 compounds were identified using Rxi-5MS and 78 using HP-INNOWAX. Our results revealed that the oil was mainly composed of five categories of compound: oxygenated monoterpenes (28.76-78.10%), oxygenated sesquiterpenes (4.27-38.06%), sesquiterpenes (3.22-11.57%), fatty hydrocarbons (1.65-9.81%) and monoterpenes (0-3.32%). The major constituents are alpha-thujone, beta-thujone, cis-sabinol, Sabinyl acetate and (-)-neointermedeol.However, the essential oil composition in the published literature differs significantly. Therefore, a cluster analysis was carried out using the top ten compositions in the reported literature as well as this study, using Minitab software. To provide detailed information on plant origin, the ITS1-5.8s-ITS2 region was amplified and sequenced (Accession No. MF668250). Besides, in order to provide a macroscopic view of the chemical composition, the biosynthetic pathway of the main components was summarized according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and the published literatures.
Relationship between chemotypic and genetic diversity of natural populations of Artemisia herba-alba Asso growing wild in Tunisia.[Pubmed:29421510]
Phytochemistry. 2018 Apr;148:48-56.
A total of 80 individuals collected from eight populations growing wild in different geographic zones were considered to assess the intraspecific variability of essential oil composition, genetic diversity and population structure of Artemisia herba-alba. The essential oil composition varied significantly between populations. Essential oil profiles were classified into four chemotypes (trans-Sabinyl acetate, alpha-thujone/trans-Sabinyl acetate, camphor and alpha-thujone/camphor/beta-thujone). Despite significant correlation between the amount of some essential oil compounds and a set of climatic data, the global chemical divergence among populations was not related to their bioclimatic and geographic appurtenances. A high level of genetic diversity within populations was revealed either with RAPD and ISSR markers (Na=1.67, PPL=66.5%, H=0.26, I=0.38 and Na=1.7, PPL=69.8%, H=0.26, I=0.38, respectively). The level of genetic diversity varied across populations and chemotypes. Populations from the alpha-thujone/trans-Sabinyl acetate chemotype exhibited the highest genetic diversity as revealed by the RAPD markers. However, populations from alpha-thujone/camphor/beta-thujone chemotype showed the important genetic variation determined by ISSR markers. A significant genetic differentiation among populations and among chemotypes was detected. The combined analysis showed a significant correlation (r=0.484, p=.032) between the chemical and molecular markers. The PCA, performed on percentages of major oil compounds and the frequencies of polymorphic RAPD and ISSR bands, divided populations according to their chemotypic classification. Taking into consideration the current situation of A. herba-alba populations and their endangered habitats, these results are of value in order to ensure the in-situ and ex-situ conservation of this medicinal species.
Essential Oil Variability in Natural Populations of Artemisia campestris (L.) and Artemisia herba-alba (Asso) and Incidence on Antiacetylcholinesterase and Antioxidant Activities.[Pubmed:28488391]
Chem Biodivers. 2017 Jul;14(7).
The intraspecific variability of Artemisia herba-alba and A. campestris essential oils and the evaluation of their antioxidant and antiacetylcholinesterase activities were determined. Artemisia herba-alba essential oil was found rich in camphor (19.61%), alpha-thujone (19.40%), beta-thujone (9.44%), chrysanthenone (9.26%), and trans-Sabinyl acetate (8.43%). The major compounds of A. campestris essential oil were germacrene D (16.38%), beta-pinene (16.33%), and limonene (9.17%). Significant variation in the essential oil composition was observed among populations of each species. The divergence between populations was attributed to the variation of some climatic factors such as altitude, annual rainfall, winter cold stress, summer precipitation, summer drought stress, evapotranspiration, and humidity. Artemisia herba-alba and A. campestris essential oils exhibited promising antioxidant and antiacetylcholinesterase activities. The level of activity varied significantly according to the species and the essential oil. The highest scavenging activity (IC50 = 0.14 mg/ml) and the uppermost capacity to prevent beta-carotene bleaching (IC50 = 0.10 mg/ml) characterized A. campestris from population 6. A. campestris population 3 possessed the uppermost ability to reduce ferric ions (450.7 mumol Fe(2+) /g EO). The population 2 of A. campestris showed the strongest antiacetylcholinesterase activity (IC50 = 0.02 mg/ml). The variation of these activities between the essential oils was explained by their composition differences.
Chemical composition and antimicrobial activity of essential oils from Acantholippia deserticola, Artemisia proceriformis, Achillea micrantha and Libanotis buchtormensis against phytopathogenic bacteria and fungi.[Pubmed:26404704]
Nat Prod Res. 2016 Sep;30(17):1950-5.
Essential oils from aerial parts of Acantholippia deserticola, Artemisia proceriformis, Achillea micrantha and Libanotis buchtormensis were analysed by GC-MS. The major compounds identified were beta-thujone (66.5 +/- 0.2%), and trans-Sabinyl acetate (12.1 +/- 0.2%) in A. deserticola; alpha-thujone (66.9 +/- 0.4%) in A. proceriformis; 1,8-cineole (26.9 +/- 0.5%), and camphor (17.7 +/- 0.3%) in A. micrantha and cis-beta-ocimene (23.3 +/- 0.3%), and trans-beta-ocimene (18.4 +/- 0.2%) in L. buchtormensis. The oils showed a weak antimicrobial effect (MIC100 > 1.5 mg/ml) on most phytopathogens tested. A moderate antimicrobial activity (MIC100 between 0.5 and 1.5 mg/ml) was displayed by the oils of A. deserticola, A. micrantha and L. buchtormensis on Septoria tritici and by the oil of A. deserticola on Septoria glycine. The antimicrobial activity was associated to the contents of beta-thujone, trans-Sabinyl acetate and trans-sabinol. Our results indicate that the tested essential oils have little inhibitory potency not suitable for use as plant protection products against the phytopathogens assayed.
Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba.[Pubmed:25632489]
Nat Prod Commun. 2014 Dec;9(12):1799-804.
Historically, natural products have been a rich source of lead molecules in drug discovery. In particular, products to treat infectious diseases have been developed and several reports about potentialities of essential oils (EO) against Leishmania could be found. In this study, we report the chemical characterization, anti-leishmanial effects and cytotoxicity of the EO from Artemisia absinthium L. Chemical analysis revealed the EO to be composed of 18 compounds, 11 of which were identified, accounting for 64.1% of the composition. The main component of the EO was trans-Sabinyl acetate, which made up 36.7%. In vitro anti-leishmanial screening showed that the A. absinthium EO inhibited the growth of promastigotes (14.4 +/- 3.6 mug/mL) and amastigotes (13.4 +/- 2.4 mug/mL) of L. amazonensis; while cytotoxicity evaluation caused 6 fold higher values than those for the parasites. In a model of experimental cutaneous leishmaniasis in BALB/c mice, five doses of EO at 30 mg/kg by intralesional route demonstrated control of lesion size and parasite burden (p< 0.05) compared with animals treated with glucantime and untreated mice. In conclusion, in vitro and in vivo results showed the potential of EO from A. absinthium as a promising source for lead or active compounds against Leishmania, which could be explored.
Essential oil variation in wild populations of Artemisia saharae (Asteraceae) from Tunisia: chemical composition, antibacterial and antioxidant properties.[Pubmed:28510956]
Bot Stud. 2014 Dec;55(1):76.
BACKGROUND: Artemisia saharae Pomel is a new taxon of Artemisia herba-alba Asso (Asteraceae) which is endemic to Tunisia and Algeria. This shrub, commonly known as white wormwood or desert wormwood, is largely used in folk medicine and as a culinary herb. The bulks aromatic plants come from wild populations whose essential oils compositions as well as their biological properties are severely affected by several factors such as geographic conditions. Therefore, the aim of the present work is to provide more information about the influence of altitude variation on the essential oil composition, antimicrobial and antioxidant properties of Artemisia saharae growing wild in the same geographical area. RESULTS: Essential oils were extracted by hydrodistillation of leaves and flowers of the plant collected from seven different altitudes of the Baten Zamour region (southwest of Tunisia). The highest essential oil yields (2.70-2.80%) were obtained for populations of high altitudes. Seventy-five compounds, representing 92.78 to 96.95% of the total essential oils, were separated and identified. Essential oils were characterized by very high percentage of oxygenated monoterpenes (52.1-72.6%) which constituted the predominant class. From the analyzed populations, the major compounds (>7%) were alpha-thujone, beta-thujone, chrysanthenone, camphor, chrysanthenyl acetate, and Sabinyl acetate. Sabinyl acetate which was detected in some populations at relatively high percentages (7.7-10.8%) seems to be characteristic to Southern Tunisian A. saharae. The studied essential oil showed a chemical diversity depending on the population altitude as revealed by linear discriminant and cluster analyses. CONCLUSIONS: Three population groups associated with altitudinal levels were distinguished. It is worthy to note that the most discriminating compounds of chemical groups were the minor ones. Despite the high variation of essential oil compositions, the high altitude population did not affect severely the antibacterial activity against the most tested strains. Altitude seems to be an important factor influencing the yield and the chemical profile of Artemisia saharae essential oils. Knowledge of the chemical composition of essential oils in relation to environmental factors is a very important quality criterion for their marketing and contributes to their valorization as functional ingredient in food technology.
Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil.[Pubmed:25317772]
Planta Med. 2014 Dec;80(18):1698-705.
In this paper, the chemical composition and biological activity of the essential oil of Artemisia absinthium was studied. The aim of this study was to investigate the potential of ethnopharmacological uses of this plant species in the treatment of gastrointestinal diseases and wounds, and as an insect repellent. The aerial part of the plant was hydrodistilled, and the chemical composition of the essential oil was analyzed by gas chromatography and gas chromatography/mass spectrometry. Forty-seven compounds, corresponding to 94.65 % of the total oil, were identified, with the main constituents being sabinene (24.49 %), Sabinyl acetate (13.64 %), and alpha-phellandrene (10.29 %). The oil yield was 0.23 % (v/w). The antimicrobial activity of the oil was investigated against ten bacterial isolates (from patients wounds and stools) and seven American Type Culture Collection strains using a microwell dilution assay. The minimal inhibitory/bactericidal concentration of the oil ranged from < 0.08 to 2.43 mg/mL and from 0.08 to 38.80 mg/mL, respectively. The antioxidant activity of the essential oil was evaluated using 2,2-diphenyl-1-picrylhydrazil and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical-scavenging methods and assessed as significant. Skin irritation potential and acute toxicity of the oil were also investigated. Results of the skin irritant reaction showed that none of the 30 volunteers developed a positive skin irritant reaction to undiluted A. absinthium essential oil. Acute oral exposure to the essential oil did not cause mortality in the treated mice, but it did cause neurological, muscle, and gastrointestinal problems. A subchronic toxicity test on Drosophila melanogaster showed that the essential oil of A. absinthium is toxic for developing insect larvae. Starting with the concentration of 0.38 % of essential oil in medium, significant mortality of larvae exposed to the oil was noted when compared to the control. Probit analysis revealed that the LC50 value of A. absinthium essential oil for D. melanogaster larvae after 15 days of exposure was 6.31 % (49 mg/mL). The essential oil also affected the development of D. melanogaster larvae and significantly delayed achievement of the pupa stadium.
Volatile compounds from Achillea tenorii (Grande) growing in the Majella National Park (Italy).[Pubmed:25103502]
Nat Prod Res. 2014;28(20):1699-704.
This work presents the first reported phytochemical study on the hydro-distilled essential oil from Achillea tenorii (Grande), collected in the protected area of Majella National Park (Italy). The composition of the essential oil was very different from those reported for the other species of Achillea nobilis group, being constituted mainly by oxygenated monoterpenes, among which ketones, alcohols and acetates compounds were the most representative. The marker compounds of A. nobilis group were not detected while the most abundant phytoconstituents were alpha-thujone (29.7%), trans-sabinol (18.6%) and trans-Sabinyl acetate (15.7%), revealing a composition quite similar to that of Artemisia absinthium.
Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris.[Pubmed:24475951]
Chem Cent J. 2014 Jan 29;8(1):6.
BACKGROUND: A large number of essential oils is reported to have significant activity against Candida albicans. But the different chemical composition influences the degree of their activity. The intention of this study was to investigate the chemical composition and the activity against Candida albicans of volatile oils obtained from Artemisia dracunculus, A. abrotanum, A. absinthium and A. vulgaris (Asteraceae). The aim of the study was to identify new chemical compounds that have effect against C. albicans.The essential oils were obtained by hydrodistillation or extraction with dichloromethane (a new procedure we developed trying to obtain better, more separated compounds) from air dried above ground plant material and analyzed by GC-MS. Additionally commercial essential oils from the same species were tested. The Candida albicans inhibition studies were carried out by the paper disc diffusion method. RESULTS: The essential oils shared common components but presented differences in composition and showed variable antifungal activity. Davanone and derivatives thereof, compounds with silphiperfolane skeleton, estragole, davanone oil, beta-thujone, Sabinyl acetate, herniarin, cis-chrysanthenyl acetate, 1,8-cineol, and terpineol were the main components of Artemisia volatiles. CONCLUSIONS: Among the volatile fractions tested those from A. abrotanum containing davanone or silphiperfolane derivatives showed the highest antifungal activity. The in vitro tests revealed that the Artemisia oils are promising candidates for further research to develop novel anti-candida drugs.
Composition and intraspecific chemical variability of the essential oil from Artemisia herba-alba growing wild in a Tunisian arid zone.[Pubmed:21072770]
Chem Biodivers. 2010 Nov;7(11):2709-17.
The intraspecific chemical variability of essential oils (50 samples) isolated from the aerial parts of Artemisia herba-alba Asso growing wild in the arid zone of Southeastern Tunisia was investigated. Analysis by GC (RI) and GC/MS allowed the identification of 54 essential oil components. The main compounds were beta-thujone and alpha-thujone, followed by 1,8-cineole, camphor, chrysanthenone, trans-Sabinyl acetate, trans-pinocarveol, and borneol. Chemometric analysis (k-means clustering and PCA) led to the partitioning into three groups. The composition of two thirds of the samples was dominated by alpha-thujone or beta-thujone. Therefore, it could be expected that wild plants of A. herba-alba randomly harvested in the area of Kirchaou and transplanted by local farmers for the cultivation in arid zones of Southern Tunisia produce an essential oil belonging to the alpha-thujone/beta-thujone chemotype and containing also 1,8-cineole, camphor, and trans-Sabinyl acetate at appreciable amounts.
Analysis of essential oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and 13C NMR.[Pubmed:19768995]
Nat Prod Commun. 2009 Aug;4(8):1113-8.
Different techniques have been utilized to determine the composition of Artemisia absinthum (wormwood) essential oil. The oil was fractionated on a silica gel column and each fraction analyzed by GC(RI), GC-MS and 13C NMR. This allowed the identification, for the first time in A. absinthium, of two diterpenes, 9-geranyl-p-cymene and 9-geranyl-alpha-terpinene, and two homoditerpenes, 9-(15,16-dihydro-15-methylene)-geranyl-p-cymene and 9-(15,16-dihydro-15-methylene)-geranyl-alpha-terpinene. Chemical variability of A. absinthium essential oils from plants collected in the surroundings of Vilnius city over several years (1999-2007) was also shown. Chemical composition was determined by GC and GC-MS. Thujones (cis+trans, 10.2-36.3%) and trans-Sabinyl acetate (9.8-39.2%) were the two predominant constituents of almost all the investigated oils (13 out of 15 samples). The third major compound was myrcene (5.1-9.2%, in four samples), beta-pinene (5.4-10.4%, in 5), linalool (4.7% in one), trans-sabinol (6.4%, in one) and 1,8-cineole (5.2-7.1%, in two). In one oil, the prevailing components were thujones (cis+trans, 11.2%), trans-sabinene hydrate (11.0%) and trans-Sabinyl acetate (8.8%), while another sample was characterized by a large quantity of trans-Sabinyl acetate (55.2%) and the absence of thujones.
Essential oil composition of Artemisia herba-alba from southern Tunisia.[Pubmed:19384287]
Molecules. 2009 Apr 20;14(4):1585-94.
The composition of the essential oil hydrodistilled from the aerial parts of 18 individual Artemisia herba-alba Asso. plants collected in southern Tunisia was determined by GC and GCMS analysis. The oil yield varied between 0.68% v/w and 1.93% v/w. One hundred components were identified, 21 of of which are reported for the first time in Artemisia herba-alba oil. The oil contained 10 components with percentages higher than 10%. The main components were cineole, thujones, chrysanthenone, camphor, borneol, chrysanthenyl acetate, Sabinyl acetate, davana ethers and davanone. Twelve samples had monoterpenes as major components, three had sesquiterpenes as major components and the last three samples had approximately the same percentage of monoterpenes and sesquiterpenes. The chemical compositions revealed that ten samples had compositions similar to those of other Artemisia herba-alba essential oils analyzed in other countries. The remaining eight samples had an original chemical composition.
Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils.[Pubmed:18417176]
Phytochemistry. 2008 May;69(8):1732-8.
The chemical composition of essential oils isolated from aerial parts of seven wild sages from Western Canada -Artemisia absinthium L., Artemisia biennis Willd., Artemisia cana Pursh, Artemisia dracunculus L., Artemisia frigida Willd., Artemisia longifolia Nutt. and Artemisia ludoviciana Nutt., was investigated by GC-MS. A total of 110 components were identified accounting for 71.0-98.8% of the oil composition. High contents of 1,8-cineole (21.5-27.6%) and camphor (15.9-37.3%) were found in Artemisia cana, A. frigida, A. longifolia and A. ludoviciana oils. The oil of A. ludoviciana was also characterized by a high content of oxygenated sesquiterpenes with a 5-ethenyltetrahydro-5-methyl-2-furanyl moiety, of which davanone (11.5%) was the main component identified. A. absinthium oil was characterized by high amounts of myrcene (10.8%), trans-thujone (10.1%) and trans-Sabinyl acetate (26.4%). A. biennis yielded an oil rich in (Z)-beta-ocimene (34.7%), (E)-beta-farnesene (40.0%) and the acetylenes (11.0%) (Z)- and (E)-en-yn-dicycloethers. A. dracunculus oil contained predominantly phenylpropanoids such as methyl chavicol (16.2%) and methyl eugenol (35.8%). Artemisia oils had inhibitory effects on the growth of bacteria (Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis), yeasts (Candida albicans, Cryptococcus neoformans), dermatophytes (Trichophyton rubrum, Microsporum canis, and Microsporum gypseum), Fonsecaea pedrosoi and Aspergillus niger. A. biennis oil was the most active against dermatophytes, Cryptococcus neoformans, Fonsecaea pedrosoi and Aspergillus niger, and A. absinthium oil the most active against Staphylococcus strains. In addition, antioxidant (beta-carotene/linoleate model) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities were determined, and weak activities were found for these oils.
Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica all.[Pubmed:16366708]
J Agric Food Chem. 2005 Dec 28;53(26):10148-53.
The chemical composition of the essential oil from flowering tops of Achillea ligustica All. was studied. Samples were collected in different localities of Sardinia (Italy) and hydrodistilled both with Clevenger-type and with simultaneous distillation-extraction apparatus. The yields ranged between 0.88 +/- 0.06 and 0.43 +/- 0.02% (vol/dry wt). The essential oils were analyzed by GC-MS, and a total of 96 components were detected. From a qualitative point of view, irrelevant differences between samples were observed. Strong chemical variability depending on the origin of the samples was observed. The major compounds found were santolina alcohol (6.7-21.8%, for the first time detected in A. ligustica), borneol (3.4-20.8%), sabinol (2.1-15.5%), trans-Sabinyl acetate (0.9-17.6%), alpha-thujone (0.4-25.8%), and, among sesquiterpenes, viridiflorol (0.7-3.6%). No significant differences were detected between essential oils extracted by hydrodistillation and simultaneous distillation-extraction with CH2Cl2 and n-hexane. Antioxidant activity as DPPH radical scavenging activity was expressed in TEAC and ranged between 0.40 and 0.88 mmol/L. The antimicrobial and antifungal activities were investigated on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Penicillium commune, Fusarium oxysporum, Rizoctonia solani, and Aspergillus flavus, showing low activity.
Chemical composition of the essential oil of Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon.[Pubmed:15527145]
J Chromatogr A. 2004 Oct 15;1052(1-2):237-40.
The essential oil of aerial parts of Salvia multicaulis Vahl. var. simplicifolia Boiss. (Lamiaceae) growing wild in Lebanon was obtained by hydrodistillation and was analysed by GC and GC-MS. 67 compounds constituting 95.2% of the oil were identified, the major components being alpha-copaene (8.0%), alpha-pinene (6.6%), myrtenol (5.7%), trans-Sabinyl acetate (5.3%).


