SB 366791Potent, selective, competitive TRPV1 antagonist CAS# 472981-92-3 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 472981-92-3 | SDF | Download SDF |
| PubChem ID | 667594 | Appearance | Powder |
| Formula | C16H14ClNO2 | M.Wt | 287.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 39 mg/mL (135.54 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
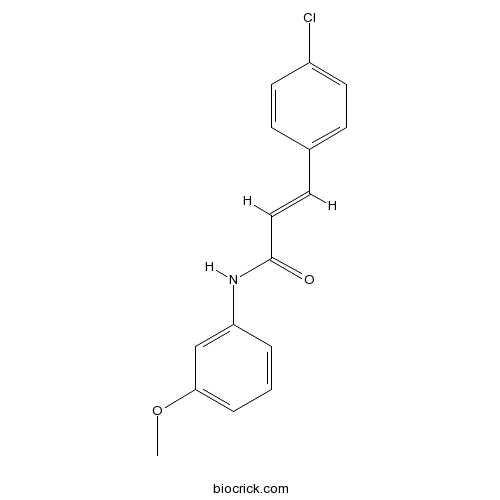
| Chemical Name | (E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)prop-2-enamide | ||
| SMILES | COC1=CC=CC(=C1)NC(=O)C=CC2=CC=C(C=C2)Cl | ||
| Standard InChIKey | RYAMDQKWNKKFHD-JXMROGBWSA-N | ||
| Standard InChI | InChI=1S/C16H14ClNO2/c1-20-15-4-2-3-14(11-15)18-16(19)10-7-12-5-8-13(17)9-6-12/h2-11H,1H3,(H,18,19)/b10-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and competitive vanilloid TRPV1 receptor antagonist (pA2 = 7.71 at hVR1); antagonizes hTRPV1 receptors activated by agonists, noxious heat, but not protons. Displays selectivity over a wide range of receptors and systems including CB1 and CB2 receptors, voltage-gated Ca2+ channels and the hyperpolarization-activated current (Ih). Also available as part of the Vanilloid TRPV1 Receptor. |

SB 366791 Dilution Calculator

SB 366791 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4752 mL | 17.3762 mL | 34.7524 mL | 69.5048 mL | 86.881 mL |
| 5 mM | 0.695 mL | 3.4752 mL | 6.9505 mL | 13.901 mL | 17.3762 mL |
| 10 mM | 0.3475 mL | 1.7376 mL | 3.4752 mL | 6.9505 mL | 8.6881 mL |
| 50 mM | 0.0695 mL | 0.3475 mL | 0.695 mL | 1.3901 mL | 1.7376 mL |
| 100 mM | 0.0348 mL | 0.1738 mL | 0.3475 mL | 0.695 mL | 0.8688 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB-366791 is a potent , competitive and selective vanilloid receptor (VR1/TRPV1) antagonist with IC50 of 5.7±1.2 nM target: VR1/TRPV1 IC 50: 5.7±1.2 nM [1] SB-366791 produced a concentration-dependent inhibition of the response to capsaicin with an apparent pKb of 7.74±0.08. Schild analysis indicated a competitive mechanism of action with a pA2 of 7.71.[1] SB-366791 showed a concentration-dependent potentiation of pH 5-induced 45Ca2+uptake in CHO cells expressing rat TRPV1 but not in untransfected cells[2]
References:
[1]. M.J. Gunthorpe et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology, 2004 Jan, 46(1):133-49.
[2]. Gavva NR et al. Proton Activation Does Not Alter Antagonist Interaction with the Capsaicin-Binding Pocket of TRPV1. Mol Pharmacol, 2005 Dec, 68(6), 1524-33.
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Butyrospermol
Catalog No.:BCN3340
CAS No.:472-28-6
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Boc-D-Ser(Bzl)-OH
Catalog No.:BCC3448
CAS No.:47173-80-8
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation.[Pubmed:16737693]
Eur J Pharmacol. 2006 Jul 1;540(1-3):73-81.
The anti-hyperalgesic effects of TRPV1 receptor antagonists are well documented in animal models of pain, however, the precise site of their action is not known. Here we have examined the effects of the selective TRPV1 antagonist SB-366791 on glutamatergic synaptic transmission in substantia gelatinosa using spinal cord slices from either control rats or animals that had undergone a peripheral inflammation induced by intraplantar injection of Freund's complete adjuvant (FCA). In control animals, SB-366791 (30 microM) had no effect on spontaneous excitatory post-synaptic currents (sEPSC) or evoked EPSCs. In slices from FCA-inflamed animals, SB-366791 decreased sEPSC frequency to 66+/-8% of control in 5/10 neurones, and decreased miniature glutamatergic EPSCs (mEPSC) frequency to 63+/-4% of control, in 6/7 neurones; with no significant effect on sEPSC or mEPSC amplitude. Dorsal root evoked EPSCs at C-fibre intensity were reduced to 72+/-6% of control by SB-366791 (30 microM) in 3/4 neurones from FCA-treated animals. In conclusion, SB-366791 inhibited glutamatergic transmission in a subset of neurones via a pre-synaptic mechanism following peripheral inflammation. We hypothesise that during peripheral inflammation spinal TRPV1 becomes tonically active, promoting the synaptic release of glutamate. These results provide evidence for a mechanism by which TRPV1 contributes to inflammatory pain and provides a basis for the understanding of the efficacy of TRPV1 antagonists.
Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist.[Pubmed:14654105]
Neuropharmacology. 2004 Jan;46(1):133-49.
Vanilloid receptor-1 (TRPV1) is a non-selective cation channel, predominantly expressed by peripheral sensory neurones, which is known to play a key role in the detection of noxious painful stimuli, such as capsaicin, acid and heat. To date, a number of antagonists have been used to study the physiological role of TRPV1; however, antagonists such as capsazepine are somewhat compromised by non-selective actions at other receptors and apparent modality-specific properties. SB-366791 is a novel, potent, and selective, cinnamide TRPV1 antagonist isolated via high-throughput screening of a large chemical library. In a FLIPR-based Ca(2+)-assay, SB-366791 produced a concentration-dependent inhibition of the response to capsaicin with an apparent pK(b) of 7.74 +/- 0.08. Schild analysis indicated a competitive mechanism of action with a pA2 of 7.71. In electrophysiological experiments, SB-366791 was demonstrated to be an effective antagonist of hTRPV1 when activated by different modalities, such as capsaicin, acid or noxious heat (50 degrees C). Unlike capsazepine, SB-366791 was also an effective antagonist vs. the acid-mediated activation of rTRPV1. With the aim of defining a useful tool compound, we also profiled SB-366791 in a wide range of selectivity assays. SB-366791 had a good selectivity profile exhibiting little or no effect in a panel of 47 binding assays (containing a wide range of G-protein-coupled receptors and ion channels) and a number of electrophysiological assays including hippocampal synaptic transmission and action potential firing of locus coeruleus or dorsal raphe neurones. Furthermore, unlike capsazepine, SB-366791 had no effect on either the hyperpolarisation-activated current (I(h)) or Voltage-gated Ca(2+)-channels (VGCC) in cultured rodent sensory neurones. In summary, SB-366791 is a new TRPV1 antagonist with high potency and an improved selectivity profile with respect to other commonly used TRPV1 antagonists. SB-366791 may therefore prove to be a useful tool to further study the biology of TRPV1.
Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1.[Pubmed:16135784]
Mol Pharmacol. 2005 Dec;68(6):1524-33.
Vanilloid receptor 1 (TRPV1) is activated by chemical ligands (e.g., capsaicin and protons) and heat. In this study, we show that (2E)-3-[2-piperidin-1-yl-6-(trifluoromethyl)pyridin-3-yl]-N-quinolin-7-ylacrylami de (AMG6880), 5-chloro-6-[(3R)-3-methyl-4-[6-(trifluoromethyl)-4-(3,4,5-trifluorophenyl)-1H-ben zimidazol-2-yl]piperazin-1-yl]pyridin-3-yl)methanol (AMG7472), and N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox amide (BCTC) are potent antagonists of rat TRPV1 activation by either capsaicin or protons (pH 5) (defined here as group A antagonists), whereas (2E)-3-(6-tert-butyl-2-methylpyridin-3-yl)-N-(1H-indol-6-yl)acrylamide (AMG0610), capsazepine, and (2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)acrylamide (SB-366791) are antagonists of capsaicin, but not proton, activation (defined here as group B antagonists). By using capsaicin-sensitive and insensitive rabbit TRPV1 channels, we show that antagonists require the same critical molecular determinants located in the transmembrane domain 3/4 region to block both capsaicin and proton activation, suggesting the presence of a single binding pocket. To determine whether the differential pharmacology is a result of proton activation-induced conformational changes in the capsaicin-binding pocket that alter group B antagonist affinities, we have developed a functional antagonist competition assay. We hypothesized that if group B antagonists bind at the same or an overlapping binding pocket of TRPV1 as group A antagonists, and proton activation does not alter the binding pocket, then group B antagonists should compete with and prevent group A antagonism of TRPV1 activation by protons. Indeed, we found that each of the group B antagonists competed with and prevented BCTC, AMG6880 or AMG7472 antagonism of rat TRPV1 activation by protons with pA2 values similar to those for blocking capsaicin, indicating that proton activation does not alter the conformation of the TRPV1 capsaicin-binding pocket. In conclusion, group A antagonists seem to lock the channel conformation in the closed state, blocking both capsaicin and proton activation.
Inhibition of C6 glioma cell proliferation by anandamide, 1-arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: variability in response and involvement of arachidonic acid.[Pubmed:12948856]
Biochem Pharmacol. 2003 Sep 1;66(5):757-67.
It has previously been shown that the endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG) inhibit the proliferation of C6 glioma cells in a manner that can be prevented by a combination of capsazepine (Caps) and cannabinoid (CB) receptor antagonists. It is not clear whether the effect of 2-AG is due to the compound itself, due to the rearrangement to form 1-arachidonoylglycerol (1-AG) or due to a metabolite. Here, it was found that the effects of 2-AG can be mimicked with 1-AG, both in terms of its potency and sensitivity to antagonism by Caps and CB receptor antagonists. In order to determine whether the effect of Caps could be ascribed to actions upon vanilloid receptors, the effect of a more selective vanilloid receptor antagonist, SB366791 was investigated. This compound inhibited capsaicin-induced Ca(2+) influx into rVR1-HEK293 cells with a pK(B) value of 6.8+/-0.3. The combination of SB366791 and CB receptor antagonists reduced the antiproliferative effect of 1-AG, confirming a vanilloid receptor component in its action. 1-AG, however, showed no direct effect on Ca(2+) influx into rVR1-HEK293 cells indicative of an indirect effect upon vanilloid receptors. Identification of the mechanism involved was hampered by a large inter-experimental variation in the sensitivity of the cells to the antiproliferative effects of 1-AG. A variation was also seen with anandamide, which was not a solubility issue, since its water soluble phosphate ester showed the same variability. In contrast, the sensitivity to methanandamide, which was not sensitive to antagonism by the combination of Caps and CB receptor antagonists, but has similar physicochemical properties to anandamide, did not vary between experiments. This variation greatly reduces the utility of these cells as a model system for the study of the antiproliferative effects of anandamide. Nevertheless, it was possible to conclude that the antiproliferative effects of anandamide were not solely mediated by either its hydrolysis to produce arachidonic acid or its CB receptor-mediated activation of phospholipase A(2) since palmitoyltrifluoromethyl ketone did not prevent the response to anandamide. The same result was seen with the fatty acid amide hydrolase inhibitor palmitoylethylamide. Increasing intracellular arachidonic acid by administration of arachidonic acid methyl ester did not affect cell proliferation, and the modest antiproliferative effect of umbelliferyl arachidonate was not prevented by a combination of Caps and CB receptor antagonists.


