AMG 487CXCR3 antagonist,potent and selective CAS# 473719-41-4 |
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- Foretinib (GSK1363089)
Catalog No.:BCC1263
CAS No.:849217-64-7
- Tivantinib (ARQ 197)
Catalog No.:BCC3688
CAS No.:905854-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 473719-41-4 | SDF | Download SDF |
| PubChem ID | 24957182 | Appearance | Powder |
| Formula | C32H28F3N5O4 | M.Wt | 603.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 41 mg/mL (67.93 mM); | ||
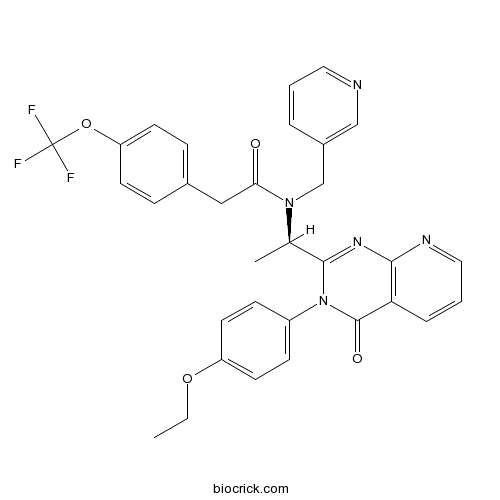
| Chemical Name | N-[(1R)-1-[3-(4-ethoxyphenyl)-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-N-(pyridin-3-ylmethyl)-2-[4-(trifluoromethoxy)phenyl]acetamide | ||
| SMILES | CCOC1=CC=C(C=C1)N2C(=O)C3=C(N=CC=C3)N=C2C(C)N(CC4=CN=CC=C4)C(=O)CC5=CC=C(C=C5)OC(F)(F)F | ||
| Standard InChIKey | WQTKNBPCJKRYPA-OAQYLSRUSA-N | ||
| Standard InChI | InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AMG 487 is an antagonist of the chemokine receptor CXCR3, which inhibits binding of 125I-IP-10 and 125I-ITAC to CXCR3 with IC50 values of 8.0 and 8.2 nM, respectively.In Vitro:AMG 487 inhibits CXCR3-mediated cell migration by the three CXCR3 chemokines (IP-10 IC50=8 nM, ITAC IC50=15 nM, and MIG IC50=36 nM). Furthermore, AMG 487 inhibits calcium mobilization in response to ITAC (IC50=5 nM)[1]. AMG487 (1 μM) develops into fewer lung metastases, and the lungs are significantly smaller than vehicle-treated lungs[2]. AMG487 abrogates proliferation/survival of C26 tumour cells[3].In Vivo:AMG 487 (0.03-10 mg/kg, s.c.) exhibits significant reduction in cellular infiltration into the lungs in a dose dependent manner[1]. AMG487 (5 mg/kg, s.c., twice daily) develops fewer metastases than that in vehicle-treated mice[2]. AMG487 (5 mg/kg, s.c.)-treated mice exhibits fewer pulmonary nodules than the control mice in both the models. AMG487 reduces the tumour volume[3]. References: | |||||

AMG 487 Dilution Calculator

AMG 487 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6568 mL | 8.2838 mL | 16.5675 mL | 33.1351 mL | 41.4188 mL |
| 5 mM | 0.3314 mL | 1.6568 mL | 3.3135 mL | 6.627 mL | 8.2838 mL |
| 10 mM | 0.1657 mL | 0.8284 mL | 1.6568 mL | 3.3135 mL | 4.1419 mL |
| 50 mM | 0.0331 mL | 0.1657 mL | 0.3314 mL | 0.6627 mL | 0.8284 mL |
| 100 mM | 0.0166 mL | 0.0828 mL | 0.1657 mL | 0.3314 mL | 0.4142 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AMG 487 is a potent and selectiveantagonist of chemokine (C-X-C motif) receptor 3 (CXCR3) with IC50 values of 8nM and 8.2nM for I-IP-10 and I-ITAC, respectively [1].
AMG 487 is an 8-azaquinazolinone, it can prevent the chemokines I-IP-10 and I-ITAC from binding to CXCR3. In the cellular assays, AMG 487 inhibits CXCR3-mediated cell migration with IC50 values of 8nM, 15nM and 36nM for I-IP-10, I-ITAC and MIG, respectively. It is also found to inhibit ITAC induced calcium mobilization with IC50 value of 5nM. In the cells, AMG 487 is converted into M1 (pyridyl N-oxide AMG 487) and M2 O-deethylated AMG 487) by CYP3A4 and CYP3A5. It is reported that the metabolite M2 can inhibit CYP3A in a competitive manner with Ki value of 0.75μM [1, 2].
References:
[1] Johnson M, Li AR, Liu J, Fu Z, Zhu L, Miao S, Wang X, Xu Q, Huang A, Marcus A, Xu F, Ebsworth K, Sablan E, Danao J, Kumer J, Dairaghi D, Lawrence C, Sullivan T, Tonn G, Schall T, Collins T, Medina J. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett. 2007 Jun 15;17(12):3339-43.
[2] Tonn GR, Wong SG, Wong SC, Johnson MG, Ma J, Cho R, Floren LC, Kersey K, Berry K, Marcus AP, Wang X, Van Lengerich B, Medina JC, Pearson PG, Wong BK. An inhibitory metabolite leads to dose- and time-dependent pharmacokinetics of (R)-N-{1-[3-(4-ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido[2,3-d]pyrimidin-2-yl]-ethyl}-N-pyridin-3-yl-methyl-2-(4-trifluoromethoxy-phenyl)-acetamide (AMG 487) in human subjects after multiple dosing. Drug Metab Dispos. 2009 Mar;37(3):502-13.
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- Citrostadienol
Catalog No.:BCN7357
CAS No.:474-40-8
- Reserpin N-oxide
Catalog No.:BCN3493
CAS No.:474-48-6
- Daucosterol
Catalog No.:BCN5531
CAS No.:474-58-8
- Campestanol
Catalog No.:BCN3890
CAS No.:474-60-2
- Campesterol
Catalog No.:BCN3181
CAS No.:474-62-4
Sequential metabolism of AMG 487, a novel CXCR3 antagonist, results in formation of quinone reactive metabolites that covalently modify CYP3A4 Cys239 and cause time-dependent inhibition of the enzyme.[Pubmed:22517972]
Drug Metab Dispos. 2012 Jul;40(7):1429-40.
CYP3A4-mediated biotransformation of (R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyrido[2,3-d]pyrimidin-2-yl)ethyl)- N-(pyridin-3-ylmethyl)-2-(4-(trifluoromethoxy)phenyl)acetamide (AMG 487) was previously shown to generate an inhibitory metabolite linked to dose- and time-dependent pharmacokinetics in humans. Although in vitro activity loss assays failed to demonstrate CYP3A4 time-dependent inhibition (TDI) with AMG 487, its M2 phenol metabolite readily produced TDI when remaining activity was assessed using either midazolam or testosterone (K(I) = 0.73-0.74 muM, k(inact) = 0.088-0.099 min(-1)). TDI investigations using an IC(50) shift method successfully produced inhibition attributable to AMG 487, but only when preincubations were extended from 30 to 90 min. The shift magnitude was approximately 3x for midazolam activity, but no shift was observed for testosterone activity. Subsequent partition ratio determinations conducted for M2 using recombinant CYP3A4 showed that inactivation was a relatively inefficient process (r = 36). CYP3A4-mediated biotransformation of [(3)H]M2 in the presence of GSH led to identification of two new metabolites, M4 and M5, which shifted focus away from M2 being directly responsible for TDI. M4 (hydroxylated M2) was further metabolized to form reactive intermediates that, upon reaction with GSH, produced isomeric adducts, collectively designated M5. Incubations conducted in the presence of [(18)O]H(2)O confirmed incorporation of oxygen from O(2) for the majority of M4 and M5 formed (>75%). Further evidence of a primary role for M4 in CYP3A4 TDI was generated by protein labeling and proteolysis experiments, in which M4 was found to be covalently bound to Cys239 of CYP3A4. These investigations confirmed a primarily role for M4 in CYP3A4 inactivation, suggesting that a more complex metabolic pathway was responsible for generation of inhibitory metabolites affecting AMG 487 human pharmacokinetics.
An inhibitory metabolite leads to dose- and time-dependent pharmacokinetics of (R)-N-{1-[3-(4-ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido[2,3-d]pyrimidin-2-yl]-ethy l}-N-pyridin-3-yl-methyl-2-(4-trifluoromethoxy-phenyl)-acetamide (AMG 487) in human subjects after multiple dosing.[Pubmed:19088267]
Drug Metab Dispos. 2009 Mar;37(3):502-13.
(R)-N-{1-[3-(4-Ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido[2,3-d]-pyrimidin-2-yl]-eth yl}-N-pyridin-3-yl-methyl-2-(4-trifluoromethoxyphenyl)-acetamide (AMG 487) is a potent and selective orally bioavailable chemokine (C-X-C motif) receptor 3 (CXCR3) antagonist that displays dose- and time-dependent pharmacokinetics in human subjects after multiple oral dosing. Although AMG 487 exhibited linear pharmacokinetics on both days 1 and 7 at the 25-mg dose, dose- and time-dependent kinetics were evident at the two higher doses. Nonlinear kinetics were more pronounced after multiple dosing. Area under the plasma concentration-time curve from 0 to 24 h [AUC((0-24 h))] increased 96-fold with a 10-fold increase in dose on day 7 compared with a 28-fold increase in AUC((0-24 h)) on day 1. These changes were correlated with time- and dose-dependent decreases in the metabolite to parent plasma concentrations, suggesting that these changes result from a decrease in the oral clearance (CL) of AMG 487 (e.g., intestinal/hepatic first-pass metabolism and systemic CL). The biotransformation of AMG 487 is dependent on CYP3A and results in the formation of two primary metabolites, a pyridyl N-oxide AMG 487 (M1) and an O-deethylated AMG 487 (M2). One of these metabolites, M2, undergoes further metabolism by CYP3A. M2 has also been demonstrated to inhibit CYP3A in a competitive (K(i)=0.75 microM) manner as well as via mechanism-based inhibition (unbound K(I)=1.4 microM, k(inact)=0.041 min(-1)). Data from this study implicate M2-mediated CYP3A mechanism-based inhibition as the proximal cause for the time-dependent pharmacokinetics of AMG 487. However, the sequential metabolism of M2, nonlinear AMG 487 pharmacokinetics, and the inability to accurately determine the role of intestinal AMG 487 metabolism complicates the correlation between M2 plasma concentrations and the time-dependent AMG 487 pharmacokinetic changes.


