Carpinontriol BCAS# 473451-73-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 473451-73-9 | SDF | Download SDF |
| PubChem ID | 132487818 | Appearance | Powder |
| Formula | C19H20O6 | M.Wt | 344.35 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
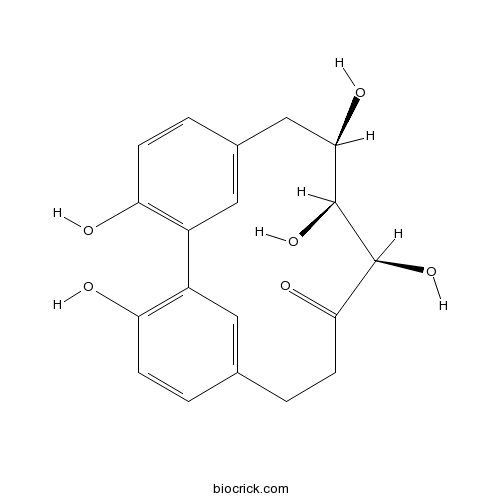
| Chemical Name | (10R,11S,12R)-3,10,11,12,17-pentahydroxytricyclo[12.3.1.12,6]nonadeca-1(17),2,4,6(19),14(18),15-hexaen-9-one | ||
| SMILES | C1CC(=O)C(C(C(CC2=CC(=C(C=C2)O)C3=C(C=CC1=C3)O)O)O)O | ||
| Standard InChIKey | HXRHVUWGMADGQP-QYZOEREBSA-N | ||
| Standard InChI | InChI=1S/C19H20O6/c20-14-4-1-10-2-6-16(22)18(24)19(25)17(23)9-11-3-5-15(21)13(8-11)12(14)7-10/h1,3-5,7-8,17-21,23-25H,2,6,9H2/t17-,18+,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Carpinontriol B has antimicrobial activity, it at 40 ug/disk caused the formation of zones of inhibition. 2. Carpinontriol B has anti-inflammatory activity, it shows considerable inhibition on the production of nitric oxide and reduces the production of interleukin-6 in dose-dependent manner in RAW 264.7 cells. |
| Targets | Antifection | NO | IL Receptor |

Carpinontriol B Dilution Calculator

Carpinontriol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.904 mL | 14.5201 mL | 29.0402 mL | 58.0804 mL | 72.6006 mL |
| 5 mM | 0.5808 mL | 2.904 mL | 5.808 mL | 11.6161 mL | 14.5201 mL |
| 10 mM | 0.2904 mL | 1.452 mL | 2.904 mL | 5.808 mL | 7.2601 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5808 mL | 1.1616 mL | 1.452 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5808 mL | 0.726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- SCH 563705
Catalog No.:BCC1933
CAS No.:473728-58-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- Citrostadienol
Catalog No.:BCN7357
CAS No.:474-40-8
- Reserpin N-oxide
Catalog No.:BCN3493
CAS No.:474-48-6
- Daucosterol
Catalog No.:BCN5531
CAS No.:474-58-8
Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities.[Pubmed:28520428]
J Nat Prod. 2017 Jun 23;80(6):1703-1713.
The methanol extract of the leafy covers of Corylus avellana, source of the Italian PGI (protected geographical indication) product "Nocciola di Giffoni", afforded two new cyclic diarylheptanoids, giffonins T and U (2 and 3), along with two known cyclic diarylheptanoids, a quinic acid, flavonoid-, and citric acid derivatives. The structures of giffonins T and U were determined as highly hydroxylated cyclic diarylheptanoids by 1D and 2D NMR experiments. Their relative configurations were assigned by a combined quantum mechanical/NMR approach, comparing the experimental (13)C/(1)H NMR chemical shift data and the related predicted values. The absolute configurations of Carpinontriol B (1) and giffonins T and U (2 and 3) were assigned by comparison of their experimental electronic circular dichroism curves with the TDDFT-predicted curves. The ability of the compounds to inhibit the lipid peroxidation induced by H2O2 and H2O2/Fe(2+) was determined by measuring the concentration of thiobarbituric acid reactive substances. Furthermore, the antimicrobial activity of the methanol extract of leafy covers of C. avellana and of the isolated compounds against the Gram-positive strains Bacillus cereus and Staphylococcus aureus and the Gram-negative strains Escherichia coli and Pseudomonas aeruginosa was evaluated. Carpinontriol B (1) and giffonin U (3) at 40 mug/disk caused the formation of zones of inhibition.
New diarylheptanoids from the stems of Carpinus cordata.[Pubmed:12350169]
J Nat Prod. 2002 Sep;65(9):1367-70.
Two new diarylheptanoids, carpinontriols A (1) and B (2), were isolated from the stems of Carpinus cordata, along with the known diarylheptanoid, casuarinondiol (3), and five known compounds, (+)-catechin (4), methyl gallate (5), methyl gallate 3-O-beta-D-glucopyranoside (6), methyl gallate 4-O-beta-D-glucopyranoside (7), and methyl gallate 3-O-beta-D-(6'-O-galloyl)-glucopyranoside (8). The structures of 1 and 2 were elucidated by spectral methods. Among the isolated compounds, compounds 4-6 and 8 showed radical-scavenging activity in the DPPH assay.


