SB 205384positive GABAA receptor modulator CAS# 160296-13-9 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160296-13-9 | SDF | Download SDF |
| PubChem ID | 197690 | Appearance | Powder |
| Formula | C17H18N2O3S | M.Wt | 330.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 100 mM in 1eq. HCl | ||
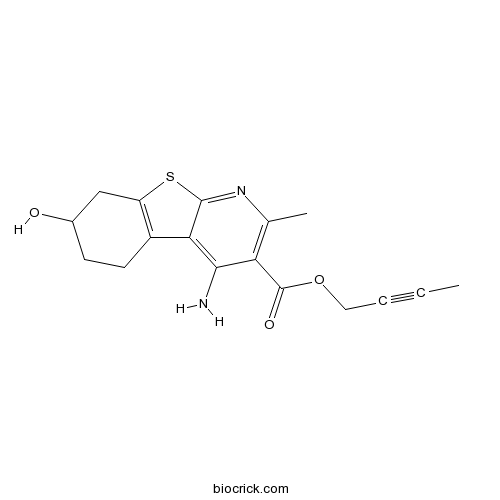
| Chemical Name | but-2-ynyl 4-amino-7-hydroxy-2-methyl-5,6,7,8-tetrahydro-[1]benzothiolo[2,3-b]pyridine-3-carboxylate | ||
| SMILES | CC#CCOC(=O)C1=C(N=C2C(=C1N)C3=C(S2)CC(CC3)O)C | ||
| Standard InChIKey | JDTZAGLGBRRCJT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18N2O3S/c1-3-4-7-22-17(21)13-9(2)19-16-14(15(13)18)11-6-5-10(20)8-12(11)23-16/h10,20H,5-8H2,1-2H3,(H2,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GABAA receptor modulator; slows current decay. Potentiates α6, α3 and α5-subunit-containing receptors (EC50 values are 280, 695 and 730 nM respectively). Displays little effect on receptors containing α1 or α2 subunits. |

SB 205384 Dilution Calculator

SB 205384 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0266 mL | 15.1332 mL | 30.2663 mL | 60.5327 mL | 75.6659 mL |
| 5 mM | 0.6053 mL | 3.0266 mL | 6.0533 mL | 12.1065 mL | 15.1332 mL |
| 10 mM | 0.3027 mL | 1.5133 mL | 3.0266 mL | 6.0533 mL | 7.5666 mL |
| 50 mM | 0.0605 mL | 0.3027 mL | 0.6053 mL | 1.2107 mL | 1.5133 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3027 mL | 0.6053 mL | 0.7567 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB-205384 is a selective and positive GABAA receptor modulator with EC50 values of 280, 695 and 730 nM on α6, α3 and α5-subunit-containing receptors, respectively [1].
g-Aminobutyric acid (GABA) acting on the GABAA receptor can inhibit synaptic transmission in the mammalian central nervous system [1]. Ligands that modulate the function of g-aminobutyric acid A (GABAA) receptor gated chloride channels have hypnotic, anaesthetic, anxiolytic, anticonvulsant and sedative actions [2].
SB-205384 can slow the decay rate on GABAA currents in oocytes expressing human GABAA subunits, which is also seen on native GABA-activated currents in rat neurons [3]. The isoforms containing the a3, a5, and a6 subunits show significantly greater enhancement of the current by SB-205384 and the receptors with the a6-subunit had the greatest sensitivity to SB-205384 [1]. In rat cerebellum granule cells, SB-205384 dramatically pro-longed the half-life for decay of the GABA response in a concentration-dependent manner. The half-life of decay was increased about 10 fold compared with control responses at the highest concentration of SB-205384 used. Also, SB-205384 did not increase the maximum current evoked by 100 mM GABA but the half-life decay was dramatically increased, as saw at the lower dose of 1 mM GABA [2].
References:
[1]. Heidelberg LS, Warren JW, Fisher JL. SB-205384 is a positive allosteric modulator of recombinant GABAA receptors containing rat α3, α5, or α6 subunit subtypes coexpressed with β3 and γ2 Subunits. J Pharmacol Exp Ther, 2013, 347(1): 235-241.
[2]. Meadows HJ, Harries MH, Thompson M, et al. Effect of SB-205384 on the decay of GABA-activated chloride currents in granule cells cultured from rat cerebellum. Br J Pharmacol, 1997, 121(7): 1334-1338.
[3]. Meadows HJ, Kumar CS, Pritchett DB, et al. SB-205384: a GABAA receptor modulator with novel mechanism of action that shows subunit selectivity. Br J Pharmacol, 1998, 123(6): 1253-1259.
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Antibiotic AB 4063B
Catalog No.:BCN1827
CAS No.:160041-33-8
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- L-368,899 hydrochloride
Catalog No.:BCC7438
CAS No.:160312-62-9
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- 3',5,5',7-Tetrahydroxyflavanone
Catalog No.:BCN1710
CAS No.:160436-10-2
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
- 12S-hydroxyandrographolide
Catalog No.:BCN4700
CAS No.:869593-50-0
- BW 723C86 hydrochloride
Catalog No.:BCC6915
CAS No.:160521-72-2
Anxiolytic-like activity of SB-205384 in the elevated plus-maze test in mice.[Pubmed:17296016]
Psicothema. 2006 Feb;18(1):100-4.
Recent studies point to a major role for alpha2-containing GABA-A receptors in modulating anxiety. However, the possible implication of GABA-A receptors containing the alpha3 subunit on anxiety is less known. The aim of this study was to examine the effects of SB-205384 (0.5-4 mg/kg, i.p.), an alpha3 subunit positive modulator of GABA-A receptor, on anxiety tested in the elevated plus-maze in male mice, using classical and ethological parameters. Mice treated with SB-205384 showed an increase in the frequency of entries and the time spent in open arms, as well as a reduction in the time spent in closed arms, as compared with the control group. A notable increase of "head-dipping" unprotected and a reduction of "stretched-attend posture" protected was also evident. These findings indicate that SB-205384 exhibits an anxiolytic-like profile in the elevated plus-maze test, suggesting that GABA-A receptors which contain the alpha3 subunit might be involved in regulation of anxiety.
Effects of SB-205384, a positive modulator of alpha3-subunit-containing GABA-A receptors, on isolation-induced aggression in male mice.[Pubmed:18206077]
Psicothema. 2008 Feb;20(1):144-7.
GABA-A receptors are involved in the control of aggressive behaviour. Various studies suggest a role for a1-containing GABA-A receptors in modulating aggression. However, the possible involvement of a3 subunit of GABA-A receptors has not been examined. In this study, we analysed the effect of SB-205384 (0.5-4 mg/kg, i.p), a positive modulator of GABA-A receptors containing a3 subunit, on agonistic behaviour elicited by isolation in male mice. Half of the mice were housed during 30 days and employed as experimental or control animals; the remainder were used as <
SB-205384: a GABA(A) receptor modulator with novel mechanism of action that shows subunit selectivity.[Pubmed:9559912]
Br J Pharmacol. 1998 Mar;123(6):1253-9.
1. SB-205384, and its (+) enantiomer (+)-SB-205384 were tested for their modulatory effects on human GABA(A) receptor subunit combinations expressed in Xenopus oocytes by electrophysiological methods. 2. The slowing of the decay rate induced by SB-205384 on native GABA-activated currents in rat neurones was also seen on GABA(A) currents in oocytes expressing human GABA(A) subunits. This temporal effect was observed for the alpha3beta2gamma2 subunit combination with little effect in subunit combinations containing either alpha1 or alpha2. 3. Potentiation of the peak amplitude of the GABA-activated currents by SB-205384 or (+)-SB-205384 was less specific for a particular subunit combination, although the greatest effect at 10 microM drug was seen on the alpha3beta2gamma2 subunit combination. 4. In contrast, zolpidem, a benzodiazepine site modulator, did not significantly slow decay rates of GABA(A) currents in oocytes expressing the alpha3beta2gamma2 subunit combination. Zolpidem, as expected, did selectively potentiate GABA-activated currents on oocytes expressing the gamma2 subunit compared to those containing the gamma1. 5. The results show that the novel kinetic modulatory profile of SB-205384 is selective for the alpha3beta2gamma2 subunit combination. This suggests that the compound is binding to a novel regulatory site on the subunit complex.
SB-205384 is a positive allosteric modulator of recombinant GABAA receptors containing rat alpha3, alpha5, or alpha6 subunit subtypes coexpressed with beta3 and gamma2 subunits.[Pubmed:23902941]
J Pharmacol Exp Ther. 2013 Oct;347(1):235-41.
Many drugs used to treat anxiety are positive modulators of GABAA receptors, which mediate fast inhibitory neurotransmission. The GABAA receptors can be assembled from a combination of at least 16 different subunits. The receptor's subunit composition determines its pharmacologic and functional properties, and subunit expression varies throughout the brain. A primary goal for new treatments targeting GABAA receptors is the production of subunit-selective modulators acting upon a discrete population of receptors. The anxiolytic 4-amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyridine-3-car boxylic acid, but-2-ynyl ester (SB-205384) is widely considered to be selective for alpha3-containing GABAA receptors. However, it has been tested only on alpha1-, alpha2-, and alpha3-containing receptors. We examined the activity of SB-205384 at recombinant receptors containing the six different alpha subunits and found that receptors containing the alpha3, alpha5, and alpha6 subunits were potentiated by SB-205384, with the alpha6 subunit conferring the greatest responsiveness. Properties associated with chimeric alpha1/alpha6 subunits suggested that multiple structural domains influence sensitivity to SB-205384. Point mutations of residues within the extracellular N-terminal domain identified a leucine residue located in loop E of the agonist binding site as an important determinant of high sensitivity to modulation. In the alpha6 subunit the identity of this residue is species-dependent, with the leucine found in rat subunits but not in human. Our results indicate that SB-205384 is not an alpha3-selective modulator, and instead acts at several GABAA receptor isoforms. These findings have implications for the side-effect profile of this anxiolytic as well as for its use in neuronal and animal studies as a marker for contribution from alpha3-containing receptors.
Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons.[Pubmed:10618148]
J Physiol. 2000 Jan 1;522 Pt 1:3-17.
We examined the maturation of GABAA receptor synapses in cortical pyramidal neurons cultured from embryonic rats. The decay kinetics of GABAA receptor-mediated miniature postsynaptic currents (mPSCs) were compared with those of responses evoked by GABA in excised membrane patches. Fast perfusion of 1 or 10 mM GABA on membrane patches evoked currents with different desensitizing time courses in young and old neurons. For neurons older than 4 days in vitro (DIV), GABAA currents had a fast component of desensitization (median approximately 3 ms) seldom seen in patches from younger neurons. In contrast, mPSCs exhibited a substantial fast component of decay at 2-4 DIV that became more prominent with further development although the median value of its time constant remained unchanged. The selective alpha3 subunit positive modulator SB-205384 had no effect on mPSCs at any time in vitro but potentiated extrasynaptic activity. This suggests that synapse maturation does not proceed by a gradual exchange of early embryonic GABAA receptor subforms for adult forms. At all ages, the kinetic properties of mPSCs were heterogeneous. This heterogeneity extended to the level of mPSCs from single neurons and may be a normal aspect of synaptic functioning. These results suggest that inhibitory synapses in developing neurons are capable of selectively capturing GABAA receptors having fast desensitization kinetics. This functional preference probably reflects the developmental turning point from an inwardly looking trophic capacity of embryonic GABAA receptors to a role concerned with information processing.
Effect of SB-205384 on the decay of GABA-activated chloride currents in granule cells cultured from rat cerebellum.[Pubmed:9257911]
Br J Pharmacol. 1997 Aug;121(7):1334-8.
1. 4-Amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyrid ine-3-carboxylic acid, but-2-ynyl ester (SB-205384) and other gamma-aminobutyric acid(A) (GABA(A)) receptor modulators were tested for their effects on GABA-activated chloride currents in rat cerebellar granule cells by use of the whole-cell patch clamp technique. 2. The major effect of SB-205384 on GABA(A)-activated current was an increase in the half-life of decay of the response once the agonist had been removed. This is in contrast to many GABA(A) receptor modulators that have previously been shown to potentiate GABA-activated currents. 3. This profile could be explained if SB-205384 stabilizes the channel in open and desensitized states so that channel closing is dramatically slowed. Such a modulatory profile may produce a novel behavioural profile in vivo.


