3',5,5',7-TetrahydroxyflavanoneCAS# 160436-10-2 |
Quality Control & MSDS
Number of papers citing our products

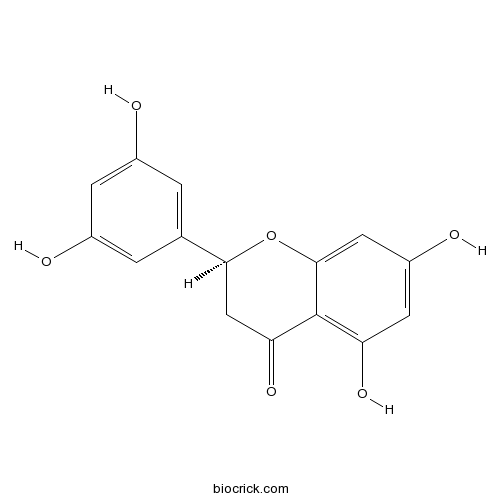
Chemical structure

3D structure
| Cas No. | 160436-10-2 | SDF | Download SDF |
| PubChem ID | 52945930 | Appearance | Powder |
| Formula | C15H12O6 | M.Wt | 288.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(3,5-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC(=CC(=C3)O)O | ||
| Standard InChIKey | AYHOUUNTAVCXBN-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C15H12O6/c16-8-1-7(2-9(17)3-8)13-6-12(20)15-11(19)4-10(18)5-14(15)21-13/h1-5,13,16-19H,6H2/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 5,7,3',5'-Tetrahydroxyflavanone has inhibitory effects on HIV-1 reverse transcriptase (RT)-associated RNase H function, it may have antiviral activity against HIV-1. 2. 5,7,3',5'-Tetrahydroxyflavanone has anti-inflammatory properties, it can inhibit nitric oxide (NO) production with IC50 values of 18.5 uM, the inhibitory effect is accompanied by dose-dependent decreases in LPS-induced nitric oxide synthase (iNOS) in RAW 264.7 cells. |
| Targets | HIV | NO | NOS |

3',5,5',7-Tetrahydroxyflavanone Dilution Calculator

3',5,5',7-Tetrahydroxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- L-368,899 hydrochloride
Catalog No.:BCC7438
CAS No.:160312-62-9
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
- 12S-hydroxyandrographolide
Catalog No.:BCN4700
CAS No.:869593-50-0
- BW 723C86 hydrochloride
Catalog No.:BCC6915
CAS No.:160521-72-2
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8-geranylflavone
Catalog No.:BCN6847
CAS No.:1605304-56-0
- Zarzissine
Catalog No.:BCN6456
CAS No.:160568-14-9
- DMP 543
Catalog No.:BCC7331
CAS No.:160588-45-4
- Villosin
Catalog No.:BCN1711
CAS No.:160598-92-5
- 1,4,6-Trihydroxy-5-methoxy-7-prenylxanthone
Catalog No.:BCN1548
CAS No.:160623-47-2
[Studies on chemical constituents of Cercis chinensis].[Pubmed:17260796]
Zhongguo Zhong Yao Za Zhi. 2006 Nov;31(21):1795-7.
OBJECTIVE: To study the chemical constituents from the aerial part of Cercis chinensis. METHOD: The constituents of C. chinensis were separated with various chromatographic techniques and their structures were elucidated by means of spectral analysis and physico-chemical properties. RESULT: Nine compounds were isolated from C. chinensis. They were identified as 3-O-methylquercetin (1), quercetin (2), (2R,3R)-3, 5, 7, 3', 5'-pentahydroxyflavan (3), 3', 5, 5', 7-tetrahydroxyflavanone (4), [+]-taxifolin (5), (2R)-naringenin (6), friedelin (7), beta-sitosterol (8), daucosterin (9). CONCLUSION: compounds 1 and 3-7 were isolated from the genus Cercis for the first time.
Hypericum hircinum L. components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities.[Pubmed:23821410]
Pathog Dis. 2013 Aug;68(3):116-24.
Among HIV-1 reverse transcriptase (RT)-associated functions, DNA polymerase and Ribonuclease H (RNase H) are both essential for HIV replication and excellent targets for drug development. While all RT inhibitors approved for therapy target the DNA polymerase activity, there is the pressing need for new RT inhibitors possibly targeting the RNase H function. In the last 20 years, many natural substances have shown antiviral activity against HIV-1, but only a few against the RNase H function. In this study, we have tested the ethanolic extracts obtained by the Hypericum hircinum L. (Hypericaceae) growing in Sardinia (Italy) on the HIV-1 RT-associated RNase H function and found that they have inhibitory effects. Active extracts were fractionated up to obtain the main components that have been isolated, tested, and identified to be betulinic acid, shikimic acid, chlorogenic acid, quercetin, 5,7,3',5'-tetrahydroxyflavanone, and 5,7,3',5'-tetrahydroxyflavanone 7-O-glucoside. Betulinic acid and 5,7,3',5'-tetrahydroxyflavanone 7-O-glucoside were active on both RT-associated activities, and betulinic acid was also active on HIV-1 mutant RTs resistant to efavirenz. Overall, our results suggest that some of these compounds inhibit the HIV-1 RT binding to an allosteric site previously described for other natural compounds and are potential leads for further drug development of a single molecules having dual inhibitory activity.
Anti-inflammatory compounds from Ampelopsis cantoniensis.[Pubmed:25924510]
Nat Prod Commun. 2015 Mar;10(3):383-5.
Many natural products have been shown to have an inhibitory effect on nitric oxide (NO), and are used as chemotherapy agents for inflammation disease. The current study was designed to evaluate the anti-inflammatory activity of chemical components from the leaves of Ampelopsis cantoniensis. Sixteen compounds (1-16) were isolated and identified. Phloretin (5) and 5,7,3',5'-tetrahydroxyflavanone (16) inhibited nitric oxide (NO) production with IC50 values of 5.2, and 18.5 muM, respectively. The inhibitory effect of compounds 5 and 16 were accompanied by dose-dependent decreases in LPS-induced nitric oxide synthase (iNOS) in RAW 264.7 cells, respectively. This study investigated the significant anti-inflammatory properties of isolated compounds from the leaves of A. cantoniensis for the first time. The findings demonstrate that A. cantoniensis could be used beneficially in the treatment of inflammation disease.


