Ro 15-4513Benzodiazepine partial inverse agonist CAS# 91917-65-6 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 91917-65-6 | SDF | Download SDF |
| PubChem ID | 5081 | Appearance | Powder |
| Formula | C15H14N6O3 | M.Wt | 326.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in ethanol and to 10 mM in DMSO | ||
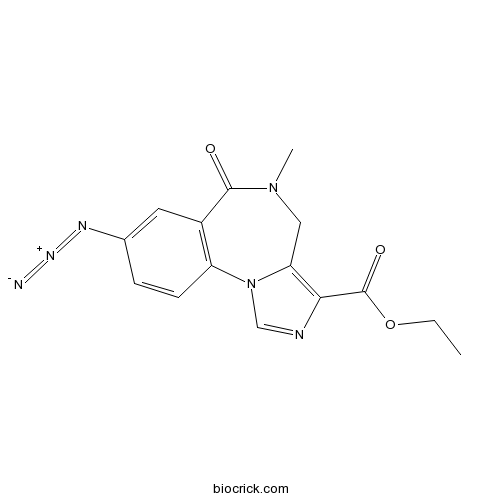
| Chemical Name | ethyl 8-azido-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate | ||
| SMILES | CCOC(=O)C1=C2CN(C(=O)C3=C(N2C=N1)C=CC(=C3)N=[N+]=[N-])C | ||
| Standard InChIKey | CFSOJZTUTOQNIA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14N6O3/c1-3-24-15(23)13-12-7-20(2)14(22)10-6-9(18-19-16)4-5-11(10)21(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity benzodiazepine ligand. Ki values are 3.1 and 5.3 nM for diazepam-insensitive (DI) and diazepam-sensitive (DS) benzodiazepine receptors respectively. Acts as partial inverse agonist at recombinant DS α1-, α2-, α3- and α5-GABAA receptors. Displays partial agonism at DI α4- and α6-GABAA receptors. Antagonizes several behavioral and neurochemical effects of ethanol. Proconvulsant and anxiogenic. |

Ro 15-4513 Dilution Calculator

Ro 15-4513 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0646 mL | 15.3229 mL | 30.6457 mL | 61.2914 mL | 76.6143 mL |
| 5 mM | 0.6129 mL | 3.0646 mL | 6.1291 mL | 12.2583 mL | 15.3229 mL |
| 10 mM | 0.3065 mL | 1.5323 mL | 3.0646 mL | 6.1291 mL | 7.6614 mL |
| 50 mM | 0.0613 mL | 0.3065 mL | 0.6129 mL | 1.2258 mL | 1.5323 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3065 mL | 0.6129 mL | 0.7661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Atrial natriuretic factor (1-28) (human, porcine)
Catalog No.:BCC5839
CAS No.:91917-63-4
- 1-Methoxyindole-3-carboxylic acid
Catalog No.:BCN3946
CAS No.:91913-76-7
- 19-[(beta-D-glucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide
Catalog No.:BCN1308
CAS No.:919120-78-8
- GPi 688
Catalog No.:BCC6091
CAS No.:918902-32-6
- TH-302
Catalog No.:BCC1998
CAS No.:918633-87-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- 5,7,4'-Trimethoxyafzelechin
Catalog No.:BCN7933
CAS No.:918428-88-3
- Cefdinir
Catalog No.:BCC3747
CAS No.:91832-40-5
- UCL 2077
Catalog No.:BCC7446
CAS No.:918311-87-2
- MK-6892
Catalog No.:BCC1767
CAS No.:917910-45-3
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
- Zatebradine hydrochloride
Catalog No.:BCC7286
CAS No.:91940-87-3
- Buergerinin B
Catalog No.:BCN4555
CAS No.:919769-83-8
- Saikosaponin H
Catalog No.:BCN7808
CAS No.:91990-63-5
- OF-1
Catalog No.:BCC5579
CAS No.:919973-83-4
- o-Acetoacetaniside
Catalog No.:BCC9107
CAS No.:92-15-9
- 6-Methylcoumarin
Catalog No.:BCN6906
CAS No.:92-48-8
- Scopoletin
Catalog No.:BCN4470
CAS No.:92-61-5
- Phenothiazine
Catalog No.:BCC4701
CAS No.:92-84-2
- 4,4'-Biphenol
Catalog No.:BCC8654
CAS No.:92-88-6
- 4-Acetylbiphenyl
Catalog No.:BCC8673
CAS No.:92-91-1
Decomposition of an aqueous solution of [11C]Ro 15-4513: implication of hydrated electrons in the radiolysis of [11C]Ro 15-4513.[Pubmed:12767396]
Nucl Med Biol. 2003 May;30(4):389-95.
An aqueous solution of [(11)C]Ro 15-4513 underwent decomposition to give a few radioactive degradation products. The major degradation product corresponded to that of authentic Ro 15-4513 with (60)Co radiolysis, which showed a molecular weight of 301 (M+H). The structure of this degradation product was estimated to have an amine group instead of an azido group on Ro 15-4513. Radiolysis of Ro 15-4513 was not suppressed by the addition of selective hydroxyl radical scavenger (MeOH, HCOO(-)), in contrast, it was suppressed effectively in the presence of a selective hydrated electron scavenger (NaNO(3)), suggesting that hydrated electrons would play an important role in the radiolysis of [(11)C]Ro 15-4513.
Selective labelling of diazepam-insensitive GABAA receptors in vivo using [3H]Ro 15-4513.[Pubmed:16184188]
Br J Pharmacol. 2005 Nov;146(6):817-25.
Classical benzodiazepines (BZs), such as diazepam, bind to GABAA receptors containing alpha1, alpha2, alpha3 or alpha5 subunits that are therefore described as diazepam-sensitive (DS) receptors. However, the corresponding binding site of GABAA receptors containing either an alpha4 or alpha6 subunit do not bind the classical BZs and are therefore diazepam-insensitive (DIS) receptors; a difference attributable to a single amino acid (histidine in alpha1, alpha2, alpha3 and alpha5 subunits and arginine in alpha4 and alpha6). Unlike classical BZs, the imidazobenzodiazepines Ro 15-4513 and bretazenil bind to both DS and DIS populations of GABAA receptors. In the present study, an in vivo assay was developed using lorazepam to fully occupy DS receptors such that [3H]Ro 15-4513 was then only able to bind to DIS receptors. When dosed i.v., [3H]Ro 15-4513 rapidly entered and was cleared from the brain, with approximately 70% of brain radioactivity being membrane-bound. Essentially all membrane binding to DS+DIS receptors could be displaced by unlabelled Ro 15-4513 or bretazenil, with respective ID50 values of 0.35 and 1.2 mg kg(-1). A dose of 30 mg kg(-1) lorazepam was used to block all DS receptors in a [3H]Ro 15-1788 in vivo binding assay. When predosed in a [3H]Ro 15-4513 binding assay, lorazepam blocked [3H]Ro 15-4513 binding to DS receptors, with the remaining binding to DIS receptors accounting for 5 and 23% of the total (DS plus DIS) receptors in the forebrain and cerebellum, respectively. The in vivo binding of [3H]Ro 15-4513 to DIS receptors in the presence of lorazepam was confirmed using alpha1H101R knock-in mice, in which alpha1-containing GABAA receptors are rendered diazepam insensitive by mutation of the histidine that confers diazepam sensitivity to arginine. In these mice, and in the presence of lorazepam, there was an increase of in vivo [3H]Ro 15-4513 binding in the forebrain and cerebellum from 4 and 15% to 36 and 59% of the total (i.e. DS plus DIS) [3H]Ro 15-4513 binding observed in the absence of lorazepam.
Rats with different thresholds for DMCM-induced clonic convulsions differ in the sleep-time of diazepam and [(3)H]-Ro 15-4513 binding.[Pubmed:22005005]
Epilepsy Res. 2012 Feb;98(2-3):216-22.
The current study investigated the possible inherent relationship between convulsions and sleep involving the GABA(A)/benzodiazepine site complex. The aim of this study was to determine if rats with high (HTR) and low (LTR) thresholds for clonic convulsions induced by DMCM, a benzodiazepine inverse agonist, differ in the following aspects: (1) sensitivity to the hypnotic effects of the GABA(A) positive allosteric modulators diazepam, pentobarbital and ethanol and (2) in the binding of [(3)H]-flunitrazepam, a benzodiazepine agonist, measured by autoradiography, and [(3)H]-Ro 15-4513, a benzodiazepine partial inverse agonist, to membranes from discrete brain regions. The LTR subgroup presented a shorter diazepam-induced sleeping time compared to that of the HTR subgroup. Biochemical assays revealed that the LTR subgroup did not differ in [(3)H]-flunitrazepam binding compared to the HTR subgroup. With respect to the binding of [(3)H]-Ro 15-4513, the LTR subgroup had higher binding in the brainstem and lower binding in the striatum compared to the HTR subgroup. These results suggest that differences in the benzodiazepine site on the GABA(A) receptor may underlie the susceptibility to DMCM-induced convulsions and sensitivity to the hypnotic effect of diazepam.
Ro 15-4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma2-type GABA(A) Receptors.[Pubmed:21270945]
Front Neurosci. 2011 Jan 20;5:3.
Ethyl alcohol (ethanol) has many molecular targets in the nervous system, its potency at these sites being low compared to those of sedative drugs. This has made it difficult to discover ethanol's binding site(s). There are two putative binding sites at gamma-aminobutyric acid (GABA) type A receptor subtypes for the proposed ethanol antagonist Ro 15-4513, the established gamma2 subunit-dependent benzodiazepine site and the recently reported delta subunit-dependent Ro 15-4513/ethanol binding site. Here, we aimed at clarifying the in vivo role of Ro 15-4513 at these two sites. We found that the antagonism of ethanol actions by Ro 15-4513 in wildtype mice was dependent on the test: an open field test showed that light sedation induced by 1.5-1.8 g/kg ethanol was sensitive to Ro 15-4513, whereas several tests for ethanol-induced anxiolytic effects showed that the ethanol-induced effects were insensitive to Ro 15-4513. Antagonism of ethanol-induced sedation by Ro 15-4513 was unaffected in GABA(A) receptor delta subunit knockout mice. By contrast, when testing the GABA(A) receptor gamma2 subunit F77I knock-in mouse line (gamma2I77 mice) with its strongly reduced affinity of the benzodiazepine sites for Ro 15-4513, we found that the ethanol-induced sedation was no longer antagonized by Ro 15-4513. Indeed, gamma2I77 mice had only a small proportion of high-affinity binding of [(3)H]Ro 15-4513 left as compared to wildtype mice, especially in the caudate-putamen and septal areas, but these residual sites are apparently not involved in ethanol antagonism. In conclusion, we found that Ro 15-4513 abolished the sedative effect of ethanol by an action on gamma2 subunit-dependent benzodiazepine sites.
Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2.[Pubmed:8913357]
Mol Pharmacol. 1996 Nov;50(5):1253-61.
We characterized modulation of the gamma-aminobutyric acid (GABA)-evoked responses of the diazepam-insensitive alpha 4 beta 2 gamma2 and alpha 6 beta 2 gamma 2 recombinant GABAA receptors. The partial agonist bretazenil potentiated the responses of both receptors with similar dose dependence but with a higher maximal enhancement at the alpha 4 beta 2 gamma 2 receptor. The bretazenil-induced potentiation was reduced by the benzodiazepine antagonist flumazenil. At a high concentration (10 microM), flumazenil was a weak potentiator of the GABA response. The partial agonist imidazenil was inactive. The imidazobenzodiazepine inverse agonist Ro 15-4513, which is known to bind with high affinity to the alpha 6 beta 2 gamma 2 receptor, potentiated the GABA responses of the alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2 receptor subtypes with similar dose dependence over the concentration range of 0.1-10 microM. Methyl-6, 7-dimethoxy-4-ethyl-beta-carboline, a beta-carboline inverse agonist, had a similar potentiating effect when tested at a concentration of 10 microM. The alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2 receptor-mediated currents had equal sensitivities to furosemide and Zn2+ ions, both of which reduced the GABA-evoked responses. The alpha 6 beta 2 gamma 2 receptor but not the alpha 4 beta 2 gamma 2 receptor exhibited a low level of spontaneous activity in the absence of GABA; this resting current could be directly potentiated by Ro 15-4513, methyl-6,7-dimethoxy-4-ethyl-beta-carboline, bretazenil and flumazenil and was blocked by picrotoxin. Thus, although the alpha 4 beta 2 gamma 2 receptors are insensitive to benzodiazepine binding site full agonists, such as diazepam, they can be modulated by certain ligands acting as partial and inverse agonists at diazepam-sensitive receptors and thereby contribute to the respective pharmacological profiles.
High affinity ligands for 'diazepam-insensitive' benzodiazepine receptors.[Pubmed:1311690]
Eur J Pharmacol. 1992 Jan 14;225(1):63-8.
Structurally diverse compounds have been shown to possess high affinities for benzodiazepine receptors in their 'diazepam-sensitive' (DS) conformations. In contrast, only the imidazobenzodiazepinone Ro 15-4513 has been shown to exhibit a high affinity for the 'diazepam-insensitive' (DI) conformation of benzodiazepine receptors. We examined a series of 1,4-diazepines containing one or more annelated ring systems for their affinities at DI and DS benzodiazepine receptors, several 1,4-diazepinone carboxylates including Ro 19-4603, Ro 16-6028 and Ro 15-3505 were found to possess high affinities (Ki approximately 2.6-20 nM) for DI. Nonetheless, among the ligands examined, Ro 15-4513 was the only substance with a DI/DS potency ratio approximately 1; other substances had ratios ranging from 13 to greater than 1000. Ligands with high to moderate affinities at DI were previously classified as partial agonists, antagonists, or partial inverse agonists at DS benzodiazepine receptors, but behaved as 'GABA neutral' (antagonist) substances at DI. The identification of several additional high affinity ligands at DI benzodiazepine receptors may be helpful in elucidating the pharmacological and physiological importance of these sites.
A selective imidazobenzodiazepine antagonist of ethanol in the rat.[Pubmed:3022383]
Science. 1986 Dec 5;234(4781):1243-7.
Ethanol, at pharmacologically relevant concentrations of 20 to 100 mM, stimulates gamma-aminobutyric (GABA) receptor-mediated uptake of 36Cl-labeled chlorine into isolated brain vesicles. One drug that acts at GABA-benzodiazepine receptors, the imidazobenzodiazepine Ro15-4513, has been found to be a potent antagonist of ethanol-stimulated 36Cl- uptake into brain vesicles, but it fails to antagonize either pentobarbital- or muscimol-stimulated 36Cl- uptake. Pretreatment of rats with Ro15-4513 blocks the anticonflict activity of low doses of ethanol (but not pentobarbital) as well as the behavioral intoxication observed with higher doses of ethanol. The effects of Ro15-4513 in antagonizing ethanol-stimulated 36Cl- uptake and behavior are completely blocked by benzodiazepine receptor antagonists. However, other benzodiazepine receptor inverse agonists fail to antagonize the actions of ethanol in vitro or in vivo, suggesting a novel interaction of Ro15-4513 with the GABA receptor-coupled chloride ion channel complex. The identification of a selective benzodiazepine antagonist of ethanol-stimulated 36Cl- uptake in vitro that blocks the anxiolytic and intoxicating actions of ethanol suggests that many of the neuropharmacologic actions of ethanol may be mediated via central GABA receptors.


