PCI 29732Inhibitor of Bruton's Tyrosine Kinase CAS# 330786-25-9 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- RN486
Catalog No.:BCC3921
CAS No.:1242156-23-5
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 330786-25-9 | SDF | Download SDF |
| PubChem ID | 22347110 | Appearance | Powder |
| Formula | C22H21N5O | M.Wt | 371.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 53 mg/mL (142.69 mM) Ethanol : 10 mg/mL (26.92 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
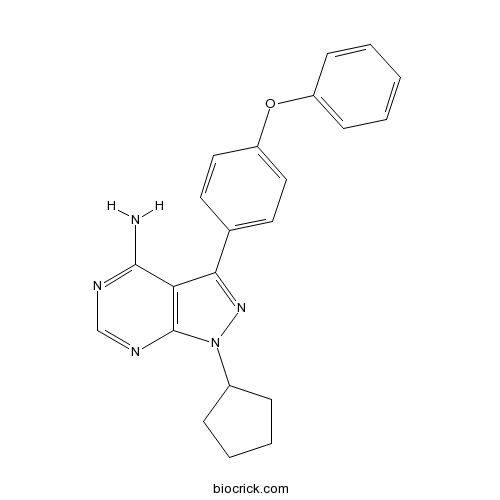
| Chemical Name | 1-cyclopentyl-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-4-amine | ||
| SMILES | C1CCC(C1)N2C3=C(C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)C(=NC=N3)N | ||
| Standard InChIKey | GMJUPMONHWAZCP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H21N5O/c23-21-19-20(26-27(16-6-4-5-7-16)22(19)25-14-24-21)15-10-12-18(13-11-15)28-17-8-2-1-3-9-17/h1-3,8-14,16H,4-7H2,(H2,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent BTK inhibitor (IC50 = 0.3 nM). Blocks B cell antigen receptor (BCR)-mediated gene expression in CD20+ B cells. |

PCI 29732 Dilution Calculator

PCI 29732 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6922 mL | 13.4611 mL | 26.9222 mL | 53.8445 mL | 67.3056 mL |
| 5 mM | 0.5384 mL | 2.6922 mL | 5.3844 mL | 10.7689 mL | 13.4611 mL |
| 10 mM | 0.2692 mL | 1.3461 mL | 2.6922 mL | 5.3844 mL | 6.7306 mL |
| 50 mM | 0.0538 mL | 0.2692 mL | 0.5384 mL | 1.0769 mL | 1.3461 mL |
| 100 mM | 0.0269 mL | 0.1346 mL | 0.2692 mL | 0.5384 mL | 0.6731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PCI 29732 is an inhibitor of Bruton’s Tyrosine Kinase with IC50 value of 8.2nM [1].
Bruton’s Tyrosine Kinase(BTK) belongs to the Tec tyrosine kinase family. It plays key roles in multiple cell signaling pathways. PCI 29732 inhibits BTK through interacting with the gatekeeper residue T474 of BTK and acts as an irreversible inhibitor. PCI 29732 has also been found to be potent inhibitors of Itk as well as other kinases from the Tec and Src family. In addition, PCI 29732 can block the transcriptional up-regulation of a panel of B-cell activation genes, thus inhibiting B-cell signaling and activation [1, 2].
References:
[1] Yan Lou, Timothy D. Owens, Andreas Kuglstatter, Rama K. Kondru and David M. Goldstein. Bruton’s tyrosine kinase inhibitors: approaches to potent and selective inhibition, preclinical and clinical evaluation for inflammatory diseases and B cell malignancies. J. Med. Chem. 2012, 55: 4539-4550.
[2] Lee A. Honigberg, Ashley M. Smith, Mint Sirisawad, Erik Verner, David Loury, Betty Chang, Shyr Li, Zhengying Pan, Douglas H. Thamm, Richard A. Miller and Joseph J. Buggy. The bruton tyrosine kinase inhibitor pci-32765 blocks b-cell activation and is efficacious in models of autoimmune disease and b-cell malignancy. PNAS. 2010, 107(29): 13075-13080.
- Avanafil
Catalog No.:BCC2288
CAS No.:330784-47-9
- Paclitaxel
Catalog No.:BCN4650
CAS No.:33069-62-4
- KH 7
Catalog No.:BCC7787
CAS No.:330676-02-3
- HCTU
Catalog No.:BCC2818
CAS No.:330645-87-9
- TCTU
Catalog No.:BCC2689
CAS No.:330641-16-2
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- H-Orn(Z)-OH
Catalog No.:BCC3003
CAS No.:3304-51-6
- Aloe-emodin-8-O-beta-D-glucopyranoside
Catalog No.:BCN1456
CAS No.:33037-46-6
- Boc-β-Ala-OH
Catalog No.:BCC3051
CAS No.:3303-84-2
- TC HSD 21
Catalog No.:BCC6228
CAS No.:330203-01-5
- SU6656
Catalog No.:BCC6392
CAS No.:330161-87-0
- Cyanidin-3-O-sambubioside chloride
Catalog No.:BCN3150
CAS No.:33012-73-6
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
Evaluation of safety and efficacy of elective PCI in patients with cardiac insufficiency.[Pubmed:28352338]
Exp Ther Med. 2017 Feb;13(2):609-613.
We analyzed the safety and the efficacy of the treatment with elective percutaneous coronary intervention (PCI) in patients with coronary heart disease complicated with cardiac insufficiency. We enrolled 217 patients diagnosed with chronic ischemic heart disease complicated with cardiac failure. According to the type of treatment they received, patients were divided into 3 groups: i) The conservative treatment group with 60 patients (they received standard medication); ii) the early PCI group with 82 cases (their condition was stabilized, surgical risk was assessed and PCI was taken as early as possible); and iii) the advanced PCI group with 75 cases (ischemic myocardium was corrected and then elective PCI was applied and for aggravated myocardial ischemia cases, PCI was applied after assessing the risk of surgery). Follow-up visits were set for approximately 3 years and clinical outcomes were compared. Our results showed that the survival time in the early PCI group was significantly prolonged and the survival rate was considerably increased during 3 years. Left ventricular ejection fraction in the early PCI group markedly increased and left ventricular end-diastolic diameter and pro-BNP level decreased significantly. The occurrence rates of perioperative complications in the early PCI group and major adverse cardiac events (MACE) during the follow-up period were significantly reduced. Quality of life scores in the early PCI group markedly improved. We concluded that in patients with coronary heart disease complicated with cardiac insufficiency, early PCI treatment was safe and effective.
Revascularization for Advanced Coronary Artery Disease in Type 2 Diabetic Patients: Choosing Wisely Between PCI and Surgery.[Pubmed:28374179]
Curr Cardiol Rep. 2017 May;19(5):37.
PURPOSE OF REVIEW: Patients with type 2 diabetes mellitus (T2DM) are at an increased risk of systemic atherosclerosis and advanced coronary artery disease (CAD). Herein, we review clinical trials comparing surgical to percutaneous revascularization in the context of the unique pathophysiology in this patient population, and seek to answer the question of optimal strategy of revascularization. RECENT FINDINGS: Early studies showed a signal towards benefit of surgical revascularization over percutaneous revascularization in this group, but there was a paucity of randomized clinical trials (RCT) to directly support this finding. The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM), a large-scale international RCT, was then undertaken and established the benefit of coronary artery bypass grafting (CABG) over percutaneous coronary intervention (PCI) in terms of mortality, myocardial infarction and repeat revascularization; CABG was inferior to PCI with regards to stroke. The quality of life and cost effectiveness also demonstrated a long-term benefit for surgery. The decision as to choice of mode of revascularization in patients with T2DM and advanced CAD depends upon a multitude of factors, including the coronary anatomy, co-morbidities and the patient's surgical risk. These factors influence the recommendation of the cardiovascular team, which should result in a balanced presentation of the short and long-term risks and benefits of either mode of revascularization to the patient and his/her family.
Active site profiling reveals coupling between domains in SRC-family kinases.[Pubmed:23143416]
Nat Chem Biol. 2013 Jan;9(1):43-50.
Protein kinases, key regulators of intracellular signal transduction, have emerged as an important class of drug targets. Chemical proteomic tools that facilitate the functional interrogation of protein kinase active sites are powerful reagents for studying the regulation of this large enzyme family and performing inhibitor selectivity screens. Here we describe a new crosslinking strategy that enables rapid and quantitative profiling of protein kinase active sites in lysates and live cells. Applying this methodology to the SRC-family kinases (SFKs) SRC and HCK led to the identification of a series of conformation-specific, ATP-competitive inhibitors that have a distinct preference for the autoinhibited forms of these kinases. Furthermore, we show that ligands that have this selectivity are able to modulate the ability of the regulatory domains of SRC and HCK to engage in intermolecular binding interactions. These studies provide insight into the regulation of this important family of tyrosine kinases.
The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy.[Pubmed:20615965]
Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13075-80.
Activation of the B-cell antigen receptor (BCR) signaling pathway contributes to the initiation and maintenance of B-cell malignancies and autoimmune diseases. The Bruton tyrosine kinase (Btk) is specifically required for BCR signaling as demonstrated by human and mouse mutations that disrupt Btk function and prevent B-cell maturation at steps that require a functional BCR pathway. Herein we describe a selective and irreversible Btk inhibitor, PCI-32765, that is currently under clinical development in patients with B-cell non-Hodgkin lymphoma. We have used this inhibitor to investigate the biologic effects of Btk inhibition on mature B-cell function and the progression of B cell-associated diseases in vivo. PCI-32765 blocked BCR signaling in human peripheral B cells at concentrations that did not affect T cell receptor signaling. In mice with collagen-induced arthritis, orally administered PCI-32765 reduced the level of circulating autoantibodies and completely suppressed disease. PCI-32765 also inhibited autoantibody production and the development of kidney disease in the MRL-Fas(lpr) lupus model. Occupancy of the Btk active site by PCI-32765 was monitored in vitro and in vivo using a fluorescent affinity probe for Btk. Active site occupancy of Btk was tightly correlated with the blockade of BCR signaling and in vivo efficacy. Finally, PCI-32765 induced objective clinical responses in dogs with spontaneous B-cell non-Hodgkin lymphoma. These findings support Btk inhibition as a therapeutic approach for the treatment of human diseases associated with activation of the BCR pathway.


