PhenothiazineCAS# 92-84-2 |
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Geniposide
Catalog No.:BCN5104
CAS No.:24512-63-8
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Imeglimin hydrochloride
Catalog No.:BCC4085
CAS No.:775351-61-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

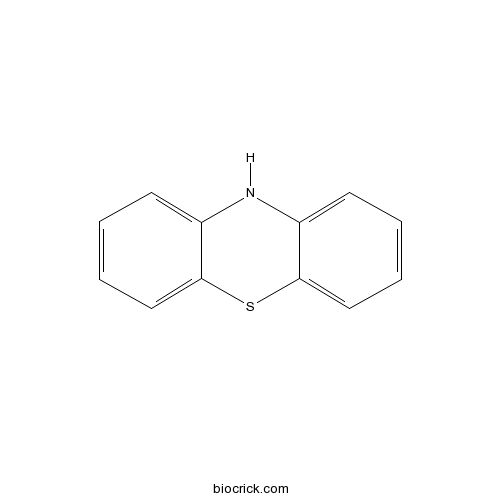
| Cas No. | 92-84-2 | SDF | Download SDF |
| PubChem ID | 7108 | Appearance | Powder |
| Formula | C12H9NS | M.Wt | 199.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | 10H-phenothiazine | ||
| SMILES | C1=CC=C2C(=C1)NC3=CC=CC=C3S2 | ||
| Standard InChIKey | WJFKNYWRSNBZNX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H9NS/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phenothiazine Dilution Calculator

Phenothiazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0183 mL | 25.0916 mL | 50.1832 mL | 100.3663 mL | 125.4579 mL |
| 5 mM | 1.0037 mL | 5.0183 mL | 10.0366 mL | 20.0733 mL | 25.0916 mL |
| 10 mM | 0.5018 mL | 2.5092 mL | 5.0183 mL | 10.0366 mL | 12.5458 mL |
| 50 mM | 0.1004 mL | 0.5018 mL | 1.0037 mL | 2.0073 mL | 2.5092 mL |
| 100 mM | 0.0502 mL | 0.2509 mL | 0.5018 mL | 1.0037 mL | 1.2546 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Phenothiazine is a dopamine-2 (D2) receptor antagonist therefore decreases the effect of dopamine in the brain.
- Scopoletin
Catalog No.:BCN4470
CAS No.:92-61-5
- 6-Methylcoumarin
Catalog No.:BCN6906
CAS No.:92-48-8
- o-Acetoacetaniside
Catalog No.:BCC9107
CAS No.:92-15-9
- OF-1
Catalog No.:BCC5579
CAS No.:919973-83-4
- Saikosaponin H
Catalog No.:BCN7808
CAS No.:91990-63-5
- Buergerinin B
Catalog No.:BCN4555
CAS No.:919769-83-8
- Zatebradine hydrochloride
Catalog No.:BCC7286
CAS No.:91940-87-3
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- Ro 15-4513
Catalog No.:BCC7230
CAS No.:91917-65-6
- Atrial natriuretic factor (1-28) (human, porcine)
Catalog No.:BCC5839
CAS No.:91917-63-4
- 1-Methoxyindole-3-carboxylic acid
Catalog No.:BCN3946
CAS No.:91913-76-7
- 4,4'-Biphenol
Catalog No.:BCC8654
CAS No.:92-88-6
- 4-Acetylbiphenyl
Catalog No.:BCC8673
CAS No.:92-91-1
- 4-Biphenylcarboxylic acid
Catalog No.:BCC8701
CAS No.:92-92-2
- Influenza Hemagglutinin (HA) Peptide
Catalog No.:BCC2563
CAS No.:92000-76-5
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
- Tenacigenoside A
Catalog No.:BCN4458
CAS No.:920502-42-7
- Schisanlactone D
Catalog No.:BCN3247
CAS No.:92051-26-8
- Schisanlactone C
Catalog No.:BCN3314
CAS No.:92051-27-9
- 4'-Methoxypuerarin
Catalog No.:BCN2901
CAS No.:92117-94-7
- ent-16-Kaurene-3beta,15beta,18-triol
Catalog No.:BCN6624
CAS No.:921211-29-2
- Fmoc-D-Lys(Boc)-OH
Catalog No.:BCC3526
CAS No.:92122-45-7
- Cysteine Protease inhibitor
Catalog No.:BCC5301
CAS No.:921625-62-9
On how electron density affects the redox stability of phenothiazine sensitizers on semiconducting surfaces.[Pubmed:28154843]
Chem Commun (Camb). 2017 Feb 23;53(17):2547-2550.
The stabilities of three organic dyes that differ only by two substituents (-OMe, -H and -Br) about the Phenothiazine donor unit were evaluated when immobilized on a semiconductor surface. All three dyes delivered modest power conversion efficiencies (PCEs) in the dye-sensitized solar cell (DSSC), but maintained 75% of their initial PCE over 300 h of sustained simulated sunlight. Electron-donating substituents increased the stability of the Phenothiazine radical unit created after light-induced charge injection into the semiconductor; however, this did not translate to higher DSSC stability, which appears to be more sensitive to the basicity of the anchoring group for this series.
Cocrystal assembled by 1,4-diiodotetrafluorobenzene and phenothiazine based on C-I...pi/N/S halogen bond and other assisting interactions.[Pubmed:28362284]
Acta Crystallogr B Struct Sci Cryst Eng Mater. 2017 Apr 1;73(Pt 2):210-216.
The halogen-bonded cocrystal of 1,4-diiodotetrafluorobenzene (1,4-DITFB) with the butterfly-shape non-planar heterocyclic compound Phenothiazine (PHT) was successfully assembled by the conventional solution-based method. X-ray single-crystal diffraction analysis reveals a 3:2 stoichiometric ratio for the cocrystal (1,4-DITFB/PHT), and the cocrystal structure is constructed via C-I...pi, C-I...N and C-I...S halogen bonds as well as other assisting interactions (e.g. C-H...F/S hydrogen bond, C-H...H-C and C-F...F-C bonds). The small shift of the 1,4-DITFB vibrational band to lower frequencies in FT-IR and Raman spectroscopies provide evidence to confirm the existence of the halogen bond. In addition, the non-planarity of the PHT molecule in the cocrystal results in PHT emitting weak phosphorescence and relatively strong delayed fluorescence. Thus, a wide range of delayed fluorescence and weak phosphorescence could play a significant role in selecting a proper pi-conjugated system to engineer functional cocrystal and luminescent materials by halogen bonds.
A comparative spectroscopic study of thiourea effect on the photophysical and molecular association behavior of various phenothiazine dyes.[Pubmed:28237658]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 May 15;179:132-143.
This paper describes the role of a structure breaking additive (thiourea) on the photophysical and molecular association of a series of Phenothiazine dyes in aqueous media using the absorption and fluorescence spectroscopic methods for the first time. The studied dyes were thionine, azure A, azure B, toluidine blue, and methylene blue. The spectral data were analyzed using DECOM program. Relevant spectral parameters in the dye solutions were estimated and discussed based on the chemical structure of the additive and excitonic treatment. The observation of spectral changes in the spectral data of the (water-additive-dye) system indicates the possible structure formation between the dye and additive molecules. However, it is found that in the high concentrations of thiourea the dimer geometries (H- and J-type dimers) are influenced by the dye-additive interactions. As a result, a competition between the dye-additive and dye-dye interactions was also observed.
Comparative analysis of triarylamine and phenothiazine sensitizer donor units in dye-sensitized solar cells.[Pubmed:28165524]
Chem Commun (Camb). 2017 Feb 16;53(15):2367-2370.
A homologous set of dyes that differ only in the donor fragments, namely Phenothiazine (PTZ) and triarylamine (TPA) units, were evaluated in dye-sensitized solar cells (DSSCs). The novel PTZ-based dye differs from the TPA-based dye in that it contains a sulfur bridge that planarizes two aromatic rings and enables higher dye loading and higher stability in the oxidized form. These positive features notwithstanding, the superior absorptivity of devices sensitized by TPA-based dyes resulted in significantly higher power conversion efficiencies (PCEs) than those sensitized by PTZ-based dyes.


