4-AcetylbiphenylCAS# 92-91-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 92-91-1 | SDF | Download SDF |
| PubChem ID | 7113 | Appearance | Powder |
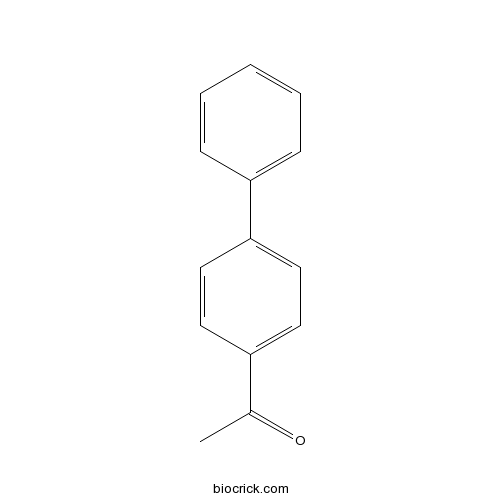
| Formula | C14H12O | M.Wt | 196 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(4-phenylphenyl)ethanone | ||
| SMILES | CC(=O)C1=CC=C(C=C1)C2=CC=CC=C2 | ||
| Standard InChIKey | QCZZSANNLWPGEA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O/c1-11(15)12-7-9-14(10-8-12)13-5-3-2-4-6-13/h2-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Acetylbiphenyl Dilution Calculator

4-Acetylbiphenyl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.102 mL | 25.5102 mL | 51.0204 mL | 102.0408 mL | 127.551 mL |
| 5 mM | 1.0204 mL | 5.102 mL | 10.2041 mL | 20.4082 mL | 25.5102 mL |
| 10 mM | 0.5102 mL | 2.551 mL | 5.102 mL | 10.2041 mL | 12.7551 mL |
| 50 mM | 0.102 mL | 0.5102 mL | 1.0204 mL | 2.0408 mL | 2.551 mL |
| 100 mM | 0.051 mL | 0.2551 mL | 0.5102 mL | 1.0204 mL | 1.2755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4,4'-Biphenol
Catalog No.:BCC8654
CAS No.:92-88-6
- Phenothiazine
Catalog No.:BCC4701
CAS No.:92-84-2
- Scopoletin
Catalog No.:BCN4470
CAS No.:92-61-5
- 6-Methylcoumarin
Catalog No.:BCN6906
CAS No.:92-48-8
- o-Acetoacetaniside
Catalog No.:BCC9107
CAS No.:92-15-9
- OF-1
Catalog No.:BCC5579
CAS No.:919973-83-4
- Saikosaponin H
Catalog No.:BCN7808
CAS No.:91990-63-5
- Buergerinin B
Catalog No.:BCN4555
CAS No.:919769-83-8
- Zatebradine hydrochloride
Catalog No.:BCC7286
CAS No.:91940-87-3
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
- Rubrisandrin A
Catalog No.:BCN3248
CAS No.:919289-30-8
- Ro 15-4513
Catalog No.:BCC7230
CAS No.:91917-65-6
- 4-Biphenylcarboxylic acid
Catalog No.:BCC8701
CAS No.:92-92-2
- Influenza Hemagglutinin (HA) Peptide
Catalog No.:BCC2563
CAS No.:92000-76-5
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
- Tenacigenoside A
Catalog No.:BCN4458
CAS No.:920502-42-7
- Schisanlactone D
Catalog No.:BCN3247
CAS No.:92051-26-8
- Schisanlactone C
Catalog No.:BCN3314
CAS No.:92051-27-9
- 4'-Methoxypuerarin
Catalog No.:BCN2901
CAS No.:92117-94-7
- ent-16-Kaurene-3beta,15beta,18-triol
Catalog No.:BCN6624
CAS No.:921211-29-2
- Fmoc-D-Lys(Boc)-OH
Catalog No.:BCC3526
CAS No.:92122-45-7
- Cysteine Protease inhibitor
Catalog No.:BCC5301
CAS No.:921625-62-9
- Neuromedin N (rat, mouse, porcine, canine)
Catalog No.:BCC5840
CAS No.:92169-45-4
- Capecitabine-2',3'-cyclic carbonate
Catalog No.:BCC8903
CAS No.:921769-65-5
Supramolecular Rotor and Translator at Work: On-Surface Movement of Single Atoms.[Pubmed:26158314]
ACS Nano. 2015 Aug 25;9(8):8394-400.
A supramolecular nanostructure composed of four 4-Acetylbiphenyl molecules and self-assembled on Au (111) was loaded with single Au adatoms and studied by scanning tunneling microscopy at low temperature. By applying voltage pulses to the supramolecular structure, the loaded Au atoms can be rotated and translated in a controlled manner. The manipulation of the gold adatoms is driven neither by mechanical interaction nor by direct electronic excitation. At the electronic resonance and driven by the tunneling current intensity, the supramolecular nanostructure performs a small amount of work of about 8 x 10(-21) J, while transporting the single Au atom from one adsorption site to the next. Using the measured average excitation time necessary to induce the movement, we determine the mechanical motive power of the device, yielding about 3 x 10(-21) W.
Palladium nanoparticles captured in microporous polymers: a tailor-made catalyst for heterogeneous carbon cross-coupling reactions.[Pubmed:20225817]
J Am Chem Soc. 2010 Apr 7;132(13):4608-13.
A new strategy based on polymerization-induced phase separation (PIPS) techniques was proposed for fabricating palladium nanoparticles (PdNPs) captured in a microporous network polymer. Pd(OAc)(2) was premixed with a monomer having a poly(amidoamine)-based dendrimer ligand, and subsequently this was thermally polymerized with an excess amount of ethylene glycol dimethacrylate under PIPS conditions. In this system, the formation of PdNPs occurred concurrently with the polymer synthesis in a one-pot process, even with no additional reducing reagent. The resultant microporous polymer was found to have a mesoporosity; the nitrogen sorption analysis gave a specific-surface area of 511 m(2) g(-1), an average pore diameter of 9.9 nm, and a total pore volume of 1.01 mL g(-1). The TEM images of the polymer revealed that the created PdNPs were very small with a diameter of mainly ca. 2.0 nm; the high-resolution images were lattice-resolvable, showing the crystalline nature of the PdNPs (Pd(111) facets). Catalytic performances of the PdNP-containing microporous polymers were investigated for a heterogeneous Suzuki-Miyaura reaction of 4'-bromoacetophenone and phenylboronic acid in water. In the presence of 10(-2) molar equiv of the polymer, the reaction efficiently proceeded at 80 degrees C and gave the desired product, 4-Acetylbiphenyl, in >90% yield after 2 h. On the basis of the ICP-AES analysis, the Pd content released into the solution phase was estimated to be only 0.27% of the initial charge. Thereby, this polymer was successfully recovered by simple filtration and reused with only a minimal loss of activity (yield >90% even at the eighth run). When the catalytic reaction was examined with a low amount of the polymer catalyst, the turnover number (TON) reached 8.5 x 10(4) while maintaining a good yield. Finally, the dendrimer template effect of the polymer catalyst was discussed by referring to the catalytic performances of a control polymer prepared with nonintegrated ligand monomers.
Docking studies and anti-inflammatory activity of beta-hydroxy-beta-arylpropanoic acids.[Pubmed:18463569]
Molecules. 2008 Mar 18;13(3):603-15.
The article describes a two-step synthesis of diastereomeric 3-hydroxy-2-methyl-3-(4-biphenylyl)butanoic acids. In the first step an intermediate alpha-bromo propanoic acid 1-ethoxyethyl ester was synthesized. The second step is a new modified Reformatsky reaction in presence of Zn in tetrahydrofuran (THF) at -5 to 10 degrees C between the previously synthesized intermediate and 4-Acetylbiphenyl. Synthesis of the other studied beta-hydroxy-beta-arylpropanoic acids has already been reported. These beta-hydroxy-beta-arylpropanoic acids belong to the arylpropanoic acid class of compounds, structurally similar to the NSAIDs such as ibuprofen. The anti-inflammatory activity and gastric tolerability of the synthesized compounds were evaluated. Molecular docking experiments were carried out to identify potential COX-2 inhibitors among the beta-hydroxy-beta-aryl-alkanoic acids class. The results indicate that all compounds possess significant anti-inflammatory activity after oral administration and that the compounds 2-(9-(9-hydroxy-fluorenyl))-2-methylpropanoic acid (5) and 3-hydroxy-3,3-diphenyl-propanoic acid (3) possess the strongest anti-inflammatory activity, comparable to that of ibuprofen, a standard NSAID,and that none of tested substances or ibuprofen produced any significant gastric lesions.


