Qc 1Threonine dehydrogenase inhibitor CAS# 403718-45-6 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Ac-LEHD-AFC
Catalog No.:BCC2359
CAS No.:210345-03-2
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 403718-45-6 | SDF | Download SDF |
| PubChem ID | 5000754 | Appearance | Powder |
| Formula | C23H16F3N3O2S | M.Wt | 455.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
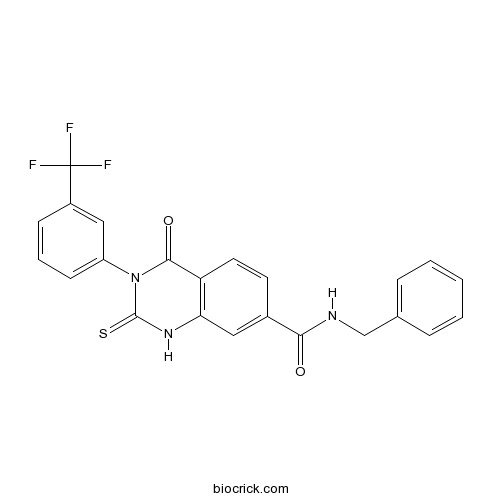
| Chemical Name | N-benzyl-4-oxo-2-sulfanylidene-3-[3-(trifluoromethyl)phenyl]-1H-quinazoline-7-carboxamide | ||
| SMILES | C1=CC=C(C=C1)CNC(=O)C2=CC3=C(C=C2)C(=O)N(C(=S)N3)C4=CC=CC(=C4)C(F)(F)F | ||
| Standard InChIKey | IFNVTSPDMUUAFY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H16F3N3O2S/c24-23(25,26)16-7-4-8-17(12-16)29-21(31)18-10-9-15(11-19(18)28-22(29)32)20(30)27-13-14-5-2-1-3-6-14/h1-12H,13H2,(H,27,30)(H,28,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible, non-competitive inhibitor of threonine dehydrogenase (TDH) (IC50 ~500 nM). Induces autophagy and inhibits cell proliferation in mouse embryonic stem (ES) cells. |

Qc 1 Dilution Calculator

Qc 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1956 mL | 10.9782 mL | 21.9563 mL | 43.9126 mL | 54.8908 mL |
| 5 mM | 0.4391 mL | 2.1956 mL | 4.3913 mL | 8.7825 mL | 10.9782 mL |
| 10 mM | 0.2196 mL | 1.0978 mL | 2.1956 mL | 4.3913 mL | 5.4891 mL |
| 50 mM | 0.0439 mL | 0.2196 mL | 0.4391 mL | 0.8783 mL | 1.0978 mL |
| 100 mM | 0.022 mL | 0.1098 mL | 0.2196 mL | 0.4391 mL | 0.5489 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Isoneobavaisoflavone
Catalog No.:BCN3195
CAS No.:40357-43-5
- p-Menth-1-ene-3,6-diol
Catalog No.:BCN5454
CAS No.:4031-55-4
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- Deacetylcinobufagin
Catalog No.:BCN2720
CAS No.:4026-95-3
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Ethyl ferulate
Catalog No.:BCN1257
CAS No.:4046-02-0
CCL2 and CCL5 driven attraction of CD172a(+) monocytic cells during an equine herpesvirus type 1 (EHV-1) infection in equine nasal mucosa and the impact of two migration inhibitors, rosiglitazone (RSG) and quinacrine (QC).[Pubmed:28241864]
Vet Res. 2017 Feb 27;48(1):14.
Equine herpesvirus type 1 (EHV-1) causes respiratory disease, abortion and neurological disorders in horses. Besides epithelial cells, CD172a(+) monocytic cells become infected with EHV-1 in the respiratory mucosa and transport the virus from the apical side of the epithelium to the lamina propria en route to the lymph and blood circulation. Whether CD172a(+) monocytic cells are specifically recruited to the infection sites in order to pick up virus is unknown. In our study, equine nasal mucosa explants were inoculated with EHV-1 neurological strains 03P37 and 95P105 or the non-neurological strains 97P70 and 94P247 and the migration of monocytic cells was examined by immunofluorescence. Further, the role of monokines CCL2 and CCL5 was determined and the effect of migration inhibitors rosiglitazone (RSG) or quinacrine was analyzed. It was shown that with neurological strains but not with the non-neurological strains, CD172a(+) cells specifically migrated towards EHV-1 infected regions and that CCL2 and CCL5 were involved. CCL2 started to be expressed in infected epithelial cells at 24 h post-incubation (hpi) and CCL5 at 48 hpi, which corresponded with the CD172a(+) migration. RSG treatment of EHV-1-inoculated equine nasal mucosa had no effect on the virus replication in the epithelium, but decreased the migration of CD172a(+) cells in the lamina propria. Overall, these findings bring new insights in the early pathogenesis of EHV-1 infections, illustrate differences between neurological and non-neurological strains and show the way for EHV-1 treatment.
Nature of E2X2 sigma(4c-6e) of the X---E-E---X type at naphthalene 1,8-positions and model, elucidated by X-ray crystallographic analysis and QC calculations with the QTAIM approach.[Pubmed:28362291]
Acta Crystallogr B Struct Sci Cryst Eng Mater. 2017 Apr 1;73(Pt 2):265-275.
The nature of E2X2 sigma(4c-6e) of the X-*-E-*-E-*-X type is elucidated for 1-(8-XC10H6)E-E(C10H6X-8')-1' [(1) E, X = S, Cl; (2) S, Br; (3) Se, Cl; (4) Se, Br] after structural determination of (1), (3) and (4), together with model A [MeX---E(H)-E(H)---XMe (E = S and Se; X = Cl and Br)]. The quantum theory of atoms-in-molecules dual functional analysis (QTAIM-DFA) is applied. The total electron energy densities Hb(rc) are plotted versus Hb(rc) - Vb(rc)/2 for the interactions at the bond critical points (BCPs; *), where Vb(rc) show the potential energy densities at the BCPs. Data for the perturbed structures around the fully optimized structures are employed for the plots, in addition to those of the fully optimized structures. The plots were analysed using the polar coordinate (R, theta) representation of the data of the fully optimized structures. Data containing the perturbed structures were analysed by (thetap, kappap), where thetap corresponds to the tangent line of the plot and kappap is the curvature. Whereas (R, theta) shows the static nature, (thetap, kappap) represents the dynamic nature of interactions. E-*-E are all classified as shared shell (S) interactions for (1)-(4) and as weak covalent (Cov-w) in nature (S/Cov-w). The nature of pure CS (closed shell)/typical-HB (hydrogen bond) with no covalency is predicted for E-*-X in (1) and (3), regular CS/typical-HB nature with covalency is predicted for (4), and an intermediate nature is predicted for (2). The NBO energies evaluated for E-*-X in (1)-(4) are substantially larger than those in model A due the shortened length at the naphthalene 1,8-positions. The nature of E2X2 of sigma(4c-6e) is well elucidated via QTAIM-DFA.
Should I repeat my 1:2s QC rejection?[Pubmed:22357876]
Clin Chem. 2012 May;58(5):925-9.
BACKGROUND: Repeating a QC that is outside 2SD from the mean (1:2s rule) appears to be a common practice. Although this form of repeat sampling is frowned on by many, the comparative power of the approach has not been formally evaluated. METHODS: We computed power functions mathematically and by computer simulation for 4 different 1:2s repeat-sampling strategies, as well as the 1:2s rule, the 1:3s rule, and 2 common QC multirules. RESULTS: The false-rejection rates for the repeat-sampling strategies were similarly low to those of the 1:3s QC rule. The error detection rates for the repeat-sampling strategies approached those of the 1:2s QC rule for moderate to large out-of-control error conditions. In most cases, the power of the repeat-sampling strategies was superior to the power of the QC multirules we evaluated. The increase in QC utilization rate ranged from 4% to 13% for the repeat-sampling strategies investigated. CONCLUSIONS: The repeat-sampling strategies provide an effective tactic to take advantage of the desirable properties of both the 1:2s and 1:3s QC rules. Additionally, the power of the repeat-sampling strategies compares favorably with the power of 2 common QC multirules. These improvements come with a modest increase in the average number of controls tested.
Linear Four-Chalcogen Interactions in Radical Cationic and Dicationic Dimers of 1,5-(Dichalcogena)canes: Nature of the Interactions Elucidated by QTAIM Dual Functional Analysis with QC Calculations.[Pubmed:28257204]
J Phys Chem A. 2017 Mar 30;121(12):2482-2496.
The dynamic and static nature of extended hypervalent interactions of the (B)E...(A)E...(A)E...(B)E type are elucidated for four center-seven electron interactions (4c-7e) in the radical cationic dimers (1.(+)) and 4c-6e in the dicationic dimers (1(2+)) of 1,5-(dichalcogena)canes (2: (A)E(CH2CH2CH2)2(B)E: (A)E, (B)E = S, Se, Te, and O). The quantum theory of atoms-in-molecules dual functional analysis (QTAIM-DFA) is applied for the analysis. Total electron energy densities Hb(rc) are plotted versus Hb(rc) - Vb(rc)/2 [= (variant Planck's over 2pi(2)/8m)nabla(2)rhob(rc)] at bond critical points (BCPs) of the interactions, where Vb(rc) values show potential energy densities at BCPs. Data from the fully optimized structures correspond to the static nature of the interactions. Those from the perturbed structures around the fully optimized ones are also plotted, in addition to those of the fully optimized ones, which represent the dynamic nature of interactions. The (B)E...(A)E-(A)E...(B)E interactions in 1(2+) are stronger than the corresponding ones in 1.(+), respectively. On the one hand, for 1(2+) with (A)E, (B)E = S, Se, and Te, (A)E...(A)E are all classified by the shared shell interactions and predicted to have the weak covalent nature, except for those in 1a(2+) ((A)E = (B)E = S) and 1d(2+) ((A)E = (B)E = Se), which have the nature of regular closed shell (r-CS)/trigonal bipyramidal adduct formation through charge transfer (CT-TBP). On the other hand, (A)E...(B)E are predicted to have the nature of r-CS/molecular complex formation through charge transfer for 1a(2+), 1b(2+) ((A)E = Se; (B)E = S), and 1d(2+) or r-CS/CT-TBP for 1c(2+) ((A)E = Te; (B)E = S), 1e(2+) ((A)E = Te; (B)E = Se), and 1f(2+) ((A)E = (B)E = Te). The (B)E...(A)E-(A)E...(B)E interactions in 1.(+) and 1(2+) are well-analyzed by applying QTAIM-DFA.
Targeted killing of a mammalian cell based upon its specialized metabolic state.[Pubmed:21896756]
Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15828-33.
Mouse ES cells use a mitochondrial threonine dehydrogenase (TDH) enzyme to catabolize threonine into glycine and acetyl-CoA. Measurements of mRNA abundance have given evidence that ES cells express upwards of 1,000-fold higher levels of TDH mRNA than any of seven other mouse tissues tested. When cell culture medium is deprived of threonine, ES cells rapidly discontinue DNA synthesis, arrest cell division, and eventually die. Such studies led to the conclusion that mouse ES cells exist in a threonine-dependent metabolic state. Proceeding with the assumption that the active TDH enzyme should be essential for the growth and viability of mouse ES cells, we performed a drug screen in search of specific inhibitors of the purified TDH enzyme. Such efforts led to the discovery of a class of quinazolinecarboxamide (Qc) compounds that inhibit the ability of the TDH enzyme to catabolize threonine into glycine and acetyl-CoA. Administration of Qc inhibitors of TDH to mouse ES cells impeded cell growth and resulted in the induction of autophagy. By contrast, the same chemicals failed to affect the growth of HeLa cells at concentrations 300-fold higher than that required to kill mouse ES cells. It was likewise observed that the Qc class of TDH inhibitors failed to affect the growth or viability of ES cell-derived embryoid body cells known to have extinguished TDH expression. These studies demonstrate how it is possible to kill a specific mammalian cell type on the basis of its specialized metabolic state.


