Firategrastα4β1/α4β7 integrin antagonist CAS# 402567-16-2 |
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 402567-16-2 | SDF | Download SDF |
| PubChem ID | 9935681 | Appearance | Powder |
| Formula | C27H27F2NO6 | M.Wt | 499.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SB 683699 | ||
| Solubility | DMSO : ≥ 100 mg/mL (200.20 mM) Ethanol : ≥ 50 mg/mL (100.10 mM) *"≥" means soluble, but saturation unknown. | ||
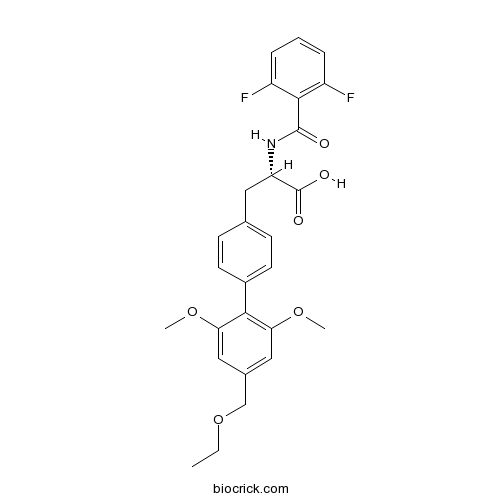
| Chemical Name | (2S)-2-[(2,6-difluorobenzoyl)amino]-3-[4-[4-(ethoxymethyl)-2,6-dimethoxyphenyl]phenyl]propanoic acid | ||
| SMILES | CCOCC1=CC(=C(C(=C1)OC)C2=CC=C(C=C2)CC(C(=O)O)NC(=O)C3=C(C=CC=C3F)F)OC | ||
| Standard InChIKey | YLFZHHDVRSYTKT-NRFANRHFSA-N | ||
| Standard InChI | InChI=1S/C27H27F2NO6/c1-4-36-15-17-13-22(34-2)24(23(14-17)35-3)18-10-8-16(9-11-18)12-21(27(32)33)30-26(31)25-19(28)6-5-7-20(25)29/h5-11,13-14,21H,4,12,15H2,1-3H3,(H,30,31)(H,32,33)/t21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Firategrast is an orally bioavailable antagonist of alpha4 beta1/alpha4 beta7 integrin. | |||||
| Targets | α4β1/α4β7 integrin | |||||

Firategrast Dilution Calculator

Firategrast Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.002 mL | 10.01 mL | 20.02 mL | 40.04 mL | 50.0501 mL |
| 5 mM | 0.4004 mL | 2.002 mL | 4.004 mL | 8.008 mL | 10.01 mL |
| 10 mM | 0.2002 mL | 1.001 mL | 2.002 mL | 4.004 mL | 5.005 mL |
| 50 mM | 0.04 mL | 0.2002 mL | 0.4004 mL | 0.8008 mL | 1.001 mL |
| 100 mM | 0.02 mL | 0.1001 mL | 0.2002 mL | 0.4004 mL | 0.5005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Firategrast is a bioavailable small-molecule antagonist of α4β1 and α4β7 integrins [1].
The integrins are a sort of transmembrane receptors that modulate the signal transduction from ECM to the cell. They are associated with a lot of diseases such as cancer, inflammation and thrombotic diseases. Since part of the integrin is exposed to the cell outside and is easy to combine with the drug, integrins are thought to be attracted targets. There are many drugs target the integrins have been designed and generated, such as abciximab, tirofiban, lamifiban and natalizumab. Among these, firategrast is a drug for the treatment of multiple sclerosis (MS) which is found to be caused by the migration of leucocytes (such as monocytes, T cells, B cells and dendritic cells) into CNS. And the integrin α4β1 is found to take participate in the migration through activating the leucocytes [1, 2].
Firategrast has a much shorter half-life than natalizumab with about 2.5 hours to 4.5 hours. It is found to inhibit the binding of the integrins to the associated ligands, including vascular cell adhesion protein 1 and mucosal addressin cell adhesion molecule 1. In CNS, firategrast treatment caused moderate decreases of total lymphocyte count, lymphocyte subset count and the ratio of CD4 to CD8. In peripheral blood, firategrast treatment resulted in the increases of total lymphocyte count, all lymphocyte subset count as well as the peripheral CD34+ early haematopoietic progenitor cell count [1, 3].
Firategrast was well tolerated at the maximum doses of 1200 mg for men and 900 mg for women. Firategrast showed no side effects, such as PML or JC-virus reactivation, at these doses. In Phase I clinical trials, the administration of firategrast significantly reduced the cumulative number of new gadolinium-enhancing lesions in patients with relapsing remitting MS [1, 3].
References:
[1] Grove R A, Shackelford S, Sopper S, et al. Leukocyte counts in cerebrospinal fluid and blood following firategrast treatment in subjects with relapsing forms of multiple sclerosis. European Journal of Neurology, 2013, 20(7): 1032-1042.
[2] Prat A, Stüve O. Firategrast—natalizumab in a pill?. The Lancet Neurology, 2012, 11(2): 120-121.
[3] Miller D H, Weber T, Grove R, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet Neurology, 2012, 11(2): 131-139.
- NSC 693868
Catalog No.:BCC7208
CAS No.:40254-90-8
- DMeOB
Catalog No.:BCC7213
CAS No.:40252-74-2
- Acetylcimigenol 3-O-alpha-L-arabinopyranside
Catalog No.:BCN1447
CAS No.:402513-88-6
- ONO-AE3-208
Catalog No.:BCC1822
CAS No.:402473-54-5
- Glycitin
Catalog No.:BCN5895
CAS No.:40246-10-4
- 5-O-Feruloylquinic acid
Catalog No.:BCN3788
CAS No.:40242-06-6
- H-Hyp-OMe.HCl
Catalog No.:BCC3248
CAS No.:40216-83-9
- H-Orn-OMe.2HCl
Catalog No.:BCC3001
CAS No.:40216-82-8
- Fmoc-β-Homo-Gln(Trt)-OH
Catalog No.:BCC2647
CAS No.:401915-55-7
- Fmoc-β-homo-Arg(Pbf)-OH
Catalog No.:BCC2649
CAS No.:401915-53-5
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- SKA 31
Catalog No.:BCC7743
CAS No.:40172-65-4
- Deacetylcinobufagin
Catalog No.:BCN2720
CAS No.:4026-95-3
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
- p-Menth-1-ene-3,6-diol
Catalog No.:BCN5454
CAS No.:4031-55-4
- Isoneobavaisoflavone
Catalog No.:BCN3195
CAS No.:40357-43-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial.[Pubmed:22226929]
Lancet Neurol. 2012 Feb;11(2):131-9.
BACKGROUND: Monoclonal antibody therapy against alpha4beta-integrin is efficacious in patients with multiple sclerosis (MS) with some safety concerns. We assessed the safety and efficacy of Firategrast, a small oral anti-alpha4beta-integrin molecule, in patients with relapsing remitting MS. METHODS: We did a multicentre, phase 2, randomised, double-blind, placebo-controlled, dose-ranging study in participants with clinically definite relapsing-remitting MS. A 24-week treatment period was followed by 12 weeks of core follow-up and 40 weeks of extended follow-up. Participants were randomly assigned, via computer-generated block randomisation in a 1:2:2:2 ratio, to receive one of four treatments twice a day: Firategrast 150 mg, Firategrast 600 mg, or Firategrast 900 mg (women) or 1200 mg (men), or placebo. Brain scans were obtained at 4-week intervals to the end of core follow-up. The primary outcome was cumulative number of new gadolinium-enhancing brain lesions during the treatment phase and was analysed using a generalised linear model with an underlying negative binomial distribution, adjusted for sex, baseline number of new gadolinium-enhancing lesions, and country. This study is registered with ClinicalTrials.gov, NCT00395317. FINDINGS: Of 343 individuals enrolled, 49 received Firategrast 150 mg, 95 received Firategrast 600 mg, 100 received Firategrast 900 mg or 1200 mg, and 99 received placebo. A 49% reduction (95% CI 21.2-67.6; p=0.0026) in the cumulative number of new gadolinium-enhancing lesions was seen for the 900 mg or 1200 mg Firategrast group (n=92, mean number of lesions 2.69 [SE 1.18]) versus the placebo group (90, 5.31 [1.18]). In the 600 mg group (86, 4.12 [SE 1.19]), a non-significant 22% reduction (95% CI -21.3 to 49.7; p=0.2657) occurred in mean number of new gadolinium-enhanced lesions relative to placebo; for the 150 mg group (47, 9.51 [SE 1.24]), a 79% increase (95% CI 4.1-308.1; p=0.0353) occurred relative to placebo. Firategrast was generally well tolerated at all doses. The frequency of all adverse events was similar across all treatment groups except for an increased rate of urinary tract infections in the high-dose Firategrast group. No cases of progressive multifocal leukoencephalopathy or evidence of reactivation of JC virus were identified. INTERPRETATION: This study showed efficacy on imaging endpoints for Firategrast at the highest dose tested, and suggests that further investigation of oral short-acting alpha4beta integrin blockade therapies is warranted. FUNDING: GlaxoSmithKline.
Leukocyte counts in cerebrospinal fluid and blood following firategrast treatment in subjects with relapsing forms of multiple sclerosis.[Pubmed:23419064]
Eur J Neurol. 2013 Jul;20(7):1032-42.
BACKGROUND AND PURPOSE: Firategrast is an orally bioavailable alpha4 beta1/alpha4 beta7 integrin antagonist designed to reduce trafficking of lymphocytes into the central nervous system (CNS). This could decrease multiple sclerosis (MS) activity, but may compromise CNS immune surveillance. We aimed to quantitate the effect of Firategrast treatment on cerebrospinal fluid (CSF) lymphocyte count and the extent/speed of recovery after its discontinuation. METHODS: Forty-six subjects with relapsing forms of MS were treated for up to 24 weeks with open-label Firategrast, 900 (females) or 1200 (males) mg twice daily. CSF and blood cell counts, and lymphocyte composition were determined using flow cytometry. RESULTS: Median (n, range) CSF lymphocyte counts (cells/microl) at weeks 0, 24, 28 and 36 were: 5.3 (44, 0.3-70.2), 3.3 (31, 0.0-99.0), 3.0 (32, 0.0-58.2) and 3.5 (29, 0.0-274.8). CD4+, CD8+ T- and CD19+ B-lymphocyte counts followed a similar pattern. Minimal changes were observed for CD3-CD16+CD56+ natural killer cells. Median CD4 : CD8 ratios were: 2.9 (41, 1.1-10.9), 2.2 (29, 0.6-5.9), 3.8 (28, 1.6-9.0) and 3.8 (21, 2.1-9.4). Blood lymphocyte counts were elevated at weeks 4 and 24, consistent with the mechanism of Firategrast, and returned to baseline when Firategrast was discontinued. There were minimal changes in CD4 : CD8 ratios. CONCLUSIONS: Firategrast treatment was associated with modest decreases in median CSF total, CD4, CD8 and CD19 lymphocyte counts. The generally small magnitude of decreases suggests that sufficient numbers of lymphocytes can access the subarachnoid space, preserving CNS immune surveillance.


