Ethyl ferulateCAS# 4046-02-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4046-02-0 | SDF | Download SDF |
| PubChem ID | 736681 | Appearance | Powder |
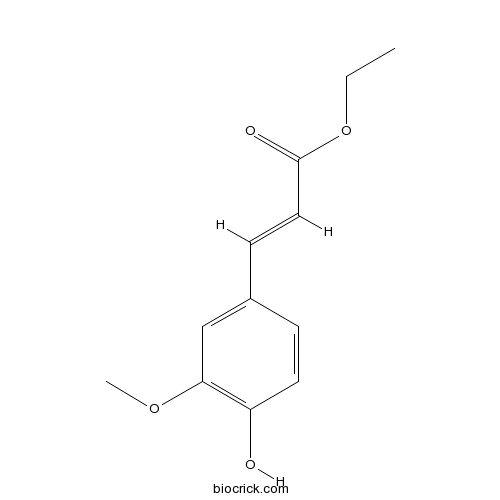
| Formula | C12H14O4 | M.Wt | 222.24 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | 28028-62-8;Ethyl (E)-ferulate | ||
| Solubility | DMSO : ≥ 100 mg/mL (449.96 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CCOC(=O)C=CC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | ATJVZXXHKSYELS-FNORWQNLSA-N | ||
| Standard InChI | InChI=1S/C12H14O4/c1-3-16-12(14)7-5-9-4-6-10(13)11(8-9)15-2/h4-8,13H,3H2,1-2H3/b7-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ethyl ferulate has anti-inflammatory property, can reduce HIV replication, it shows inhibitive effect on platelet congregation induced by ADP. It also is a potent inducer of HO-1 for the protection of brain cells against oxidative and neurodegenerative conditions. |
| Targets | HO-1 | HIV |
| In vitro | Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams.[Pubmed: 24941338]Molecules. 2014 Jun 17;19(6):8124-39.Ethyl ferulate (FAEE) has been widely studied due to its beneficial heath properties and, when incorporated in creams, shows a high sun protection capacity.
Inhibitive effect and mechanism of Ethyl ferulate on platelet congregation induced by ADP.[Reference: WebLink]Journal of the Fourth Military Medical University, 2002,23(6):537-9.To investigate the inhibitory actions of Ethyl ferulate on platelet congregate induced by ADP, and the effect of platelet intracellular calcium oscillations. |
| Cell Research | Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress.[Pubmed: 15345140]Antioxid Redox Signal. 2004 Oct;6(5):811-8.Ethyl ferulate (ethyl 4-hydroxy-3-methoxycinnamate) (EFE), the naturally occurring ester of ferulic acid, was able to induce HO-1 protein expression.
|
| Structure Identification | FEBS Lett. 1997 Nov 24;418(1-2):15-8.Protective effects of the lipophilic redox conjugate tocopheryl succinyl-ethyl ferulate on HIV replication.[Pubmed: 9414085]Previously, we demonstrated that ferulate ethyl and tocopherol reduced HIV replication.
|

Ethyl ferulate Dilution Calculator

Ethyl ferulate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4996 mL | 22.4982 mL | 44.9964 mL | 89.9928 mL | 112.491 mL |
| 5 mM | 0.8999 mL | 4.4996 mL | 8.9993 mL | 17.9986 mL | 22.4982 mL |
| 10 mM | 0.45 mL | 2.2498 mL | 4.4996 mL | 8.9993 mL | 11.2491 mL |
| 50 mM | 0.09 mL | 0.45 mL | 0.8999 mL | 1.7999 mL | 2.2498 mL |
| 100 mM | 0.045 mL | 0.225 mL | 0.45 mL | 0.8999 mL | 1.1249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bursehernin
Catalog No.:BCN3040
CAS No.:40456-51-7
- Yatein
Catalog No.:BCN5456
CAS No.:40456-50-6
- Kaempferol-3-O-glucorhamnoside
Catalog No.:BCN2830
CAS No.:40437-72-7
- Pinusolidic acid
Catalog No.:BCN5455
CAS No.:40433-82-7
- erythro-1-Phenylpropane-1,2-diol
Catalog No.:BCN6596
CAS No.:40421-52-1
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- Z-Lys(Z)-OH
Catalog No.:BCC2762
CAS No.:405-39-0
- 4'-Demethylpodophyllotoxin
Catalog No.:BCN2625
CAS No.:40505-27-9
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
- NFPS
Catalog No.:BCC7484
CAS No.:405225-21-0
- Dadahol A
Catalog No.:BCN5457
CAS No.:405281-76-7
Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress.[Pubmed:15345140]
Antioxid Redox Signal. 2004 Oct;6(5):811-8.
In the CNS, the heme oxygenase (HO) system has been reported to be active and to operate as a fundamental defensive mechanism for neurons exposed to an oxidant challenge. We have recently shown that both curcumin and caffeic acid phenethyl ester, two phenolic natural compounds, potently induce HO-1 expression and activity in rat astrocytes. We have extended our previous findings examining the effects of two other plant-derived phenolic compounds, with analogous chemical structures, in rat astrocytes and neurons. Ethyl ferulate (ethyl 4-hydroxy-3-methoxycinnamate) (EFE), the naturally occurring ester of ferulic acid, was able to induce HO-1 protein expression. Maximal expression of HO-1 mRNA and protein and a significant increase in HO activity were detected after 6 h of incubation with 15 microM EFE in astrocytes and 5 microM EFE in neurons. Higher concentrations of EFE (50 microM) caused a substantial cytotoxic effect with no change in HO-1 protein expression and activity. Exposure of astrocytes to resveratrol, a phytoalexin derived from grapes, resulted in an increase of HO-1 mRNA, but it was not able to induce HO-1 protein expression and activity. Interestingly, preincubation (12 h) of neurons with EFE resulted in an enhanced cellular resistance to glucose oxidase-mediated oxidative damage; this cytoprotective effect was considerably attenuated by zinc protoporphyrin IX, an inhibitor of HO activity. This study identifies a novel natural compound that could be used for therapeutic purposes as a potent inducer of HO-1 for the protection of brain cells against oxidative and neurodegenerative conditions.
Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams.[Pubmed:24941338]
Molecules. 2014 Jun 17;19(6):8124-39.
Ethyl ferulate (FAEE) has been widely studied due to its beneficial heath properties and, when incorporated in creams, shows a high sun protection capacity. Here we aimed to compare FAEE and its precursor, ferulic acid (FA), as free radical scavengers, inhibitors of oxidants produced by leukocytes and the alterations in rheological properties when incorporated in emulsion based creams. The cell-free antiradical capacity of FAEE was decreased compared to FA. However, FAEE was more effective regarding the scavenging of reactive oxygen species produced by activated leukocytes. Stress and frequency sweep tests showed that the formulations are more elastic than viscous. The viscoelastic features of the formulations were confirmed in the creep and recovery assay and showed that the FAEE formulation was less susceptive to deformation. Liberation experiments showed that the rate of FAEE release from the emulsion was slower compared to FA. In conclusion, FAEE is more effective than FA as a potential inhibitor of oxidative damage produced by oxidants generated by leukocytes. The rheological alterations caused by the addition of FAEE are indicative of lower spreadability, which could be useful for formulations used in restricted areas of the skin.
Protective effects of the lipophilic redox conjugate tocopheryl succinyl-ethyl ferulate on HIV replication.[Pubmed:9414085]
FEBS Lett. 1997 Nov 24;418(1-2):15-8.
Previously, we demonstrated that ferulate ethyl and tocopherol reduced HIV replication. In this study, we investigate whether the conjugation of both compounds (O-tocopheryl succinyl O-Ethyl ferulate) can increase HIV inhibition. We show here for the first time that O-tocopheryl succinyl O-Ethyl ferulate inhibits 80% of HIV replication (HIV-1 acute infection and HIV transmission), inhibits cell lipoperoxidation and prevents cellular glutathione consumption. Compared to ferulate ethyl and tocopheryl succinyl, O-tocopheryl succinyl O-Ethyl ferulate inhibits more HIV replication. This may be due in part to the great increase in the lipophilicity of this compound.


