MDL 12330A hydrochlorideadenylate cyclase inhibitor CAS# 40297-09-4 |
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
- TLQP 21
Catalog No.:BCC2405
CAS No.:869988-94-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40297-09-4 | SDF | Download SDF |
| PubChem ID | 6917816 | Appearance | Powder |
| Formula | C23H37ClN2 | M.Wt | 377.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in DMSO and to 20 mM in ethanol | ||
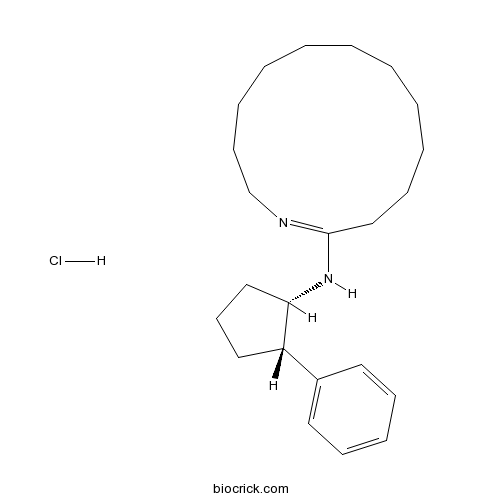
| Chemical Name | N-[(1S,2S)-2-phenylcyclopentyl]-1-azacyclotridecen-2-amine;hydrochloride | ||
| SMILES | C1CCCCCC(=NCCCCC1)NC2CCCC2C3=CC=CC=C3.Cl | ||
| Standard InChIKey | CKOPQUCSDBVAQG-VROPFNGYSA-N | ||
| Standard InChI | InChI=1S/C23H36N2.ClH/c1-2-4-6-11-18-23(24-19-12-7-5-3-1)25-22-17-13-16-21(22)20-14-9-8-10-15-20;/h8-10,14-15,21-22H,1-7,11-13,16-19H2,(H,24,25);1H/t21-,22-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of adenylyl cyclase. Also inhibits cAMP and cGMP phosphodiesterases, and blocks slow extracellular and store-operated Ca2+ entry into cells. |

MDL 12330A hydrochloride Dilution Calculator

MDL 12330A hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6524 mL | 13.2622 mL | 26.5245 mL | 53.049 mL | 66.3112 mL |
| 5 mM | 0.5305 mL | 2.6524 mL | 5.3049 mL | 10.6098 mL | 13.2622 mL |
| 10 mM | 0.2652 mL | 1.3262 mL | 2.6524 mL | 5.3049 mL | 6.6311 mL |
| 50 mM | 0.053 mL | 0.2652 mL | 0.5305 mL | 1.061 mL | 1.3262 mL |
| 100 mM | 0.0265 mL | 0.1326 mL | 0.2652 mL | 0.5305 mL | 0.6631 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1.5 X 10-5 M for histamine-stimulated adenylate cyclase [1].
RMI 12330 A is N,-(cis-2-phenylcyclopentyl) azacyclotridecan-2-imine hydrochloride, which has been reported to inhibit choleratoxin-induced intestinal hypersecretion, presumably via an inhibition of mucosal adenylate cyclase.
In vitro: RMI 12330 A at the concentration of ranging from 10 uM to 5 mM the can effectively inhibit the adenylate cyclase activity of a rat liver plasma membrane preparation. RMI 12330 A does not apply its competition on the catalytic site of the enzyme to perform its inhibitory action. The inhibition activity of RMI 12330 A upon adenylate cyclase was due to an irreversible binding with the plasma membranes [2].
Ex vivo: In the isolated work-performing heart of the guinea-pig, RMI 12330A depressed all cardiac functions with the IC50 of 1.1 X 10-6 M, including contractile force, dP/dt, dF/dt, pressures developed, stroke work and work performance. The positive inotropic response to increasing heart rate (staircase) and left atrial pressure rose became negative. Ouabain, isoprenaline and histamine, no longer had positive inotropic effects. After its depression by RMI 12330A, the cardiac function can be completely restored by increasing the perfusate calcium concentration from 2.5 mm to 4.5 and 6.5 mm [1].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Grupp G, Grupp IL, Johnson CL, Matlib MA, Rouslin W, Schwartz A, Wallick ET, Wang T, Wisler P. Effects of RMI 12330A, a new inhibitor of adenylate cyclase on myocardial function and subcellular activity. Br J Pharmacol. 1980 Nov;70(3):429-42.
[2] Guellaen G, Mahu JL, Mavier P, Berthelot P, Hanoune J. RMI 12330 A, an inhibitor of adenylate cyclase in rat liver. Biochim Biophys Acta. 1977 Oct 13;484(2):465-75.
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- Deacetylcinobufagin
Catalog No.:BCN2720
CAS No.:4026-95-3
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- NSC 693868
Catalog No.:BCC7208
CAS No.:40254-90-8
- DMeOB
Catalog No.:BCC7213
CAS No.:40252-74-2
- Acetylcimigenol 3-O-alpha-L-arabinopyranside
Catalog No.:BCN1447
CAS No.:402513-88-6
- ONO-AE3-208
Catalog No.:BCC1822
CAS No.:402473-54-5
- Glycitin
Catalog No.:BCN5895
CAS No.:40246-10-4
- 5-O-Feruloylquinic acid
Catalog No.:BCN3788
CAS No.:40242-06-6
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
- p-Menth-1-ene-3,6-diol
Catalog No.:BCN5454
CAS No.:4031-55-4
- Isoneobavaisoflavone
Catalog No.:BCN3195
CAS No.:40357-43-5
- Obtusafuran methyl ether
Catalog No.:BCN8103
CAS No.:40357-59-3
- Cannabisin H
Catalog No.:BCN3896
CAS No.:403647-08-5
- Qc 1
Catalog No.:BCC6356
CAS No.:403718-45-6
- 10058-F4
Catalog No.:BCC1050
CAS No.:403811-55-2
- HEMADO
Catalog No.:BCC7118
CAS No.:403842-38-6
- Benzyl 2-hydroxy-6-(beta-glucosyloxy)benzoate
Catalog No.:BCN7434
CAS No.:403857-21-6
- 6-Methoxykaempferol 3-O-rutinoside
Catalog No.:BCN2379
CAS No.:403861-33-6
- Pamidronate
Catalog No.:BCC4693
CAS No.:40391-99-9
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. Comparison of coupling and function.[Pubmed:10878007]
J Biol Chem. 2000 Sep 15;275(37):28562-8.
The mechanism for coupling between Ca(2+) stores and store-operated channels (SOCs) is an important but unresolved question. SOC-mediated Ca(2+) entry is complex and may reflect more than one type of channel and coupling mechanism. To assess such possible divergence the function and coupling of SOCs was compared with two other distinct yet related Ca(2+) entry mechanisms. SOC coupling in DDT(1)MF-2 smooth muscle cells was prevented by the permeant inositol 1,4,5-trisphosphate (InsP(3)) receptor blockers, 2-aminoethoxydiphenyl borate (2-APB) and xestospongin C. In contrast, Ca(2+) entry induced by S-nitrosylation and potentiated by store depletion (Ma, H-T., Favre, C. J., Patterson, R. L., Stone, M. R., and Gill, D. L. (1999) J. Biol. Chem. 274, 35318-35324) was unaffected by 2-APB, suggesting that this entry mechanism is independent of InsP(3) receptors. The cycloalkyl lactamimide, MDL-12, 330A (MDL), prevented SOC activation (IC(50) 10 micrometer) and similarly completely blocked S-nitrosylation-mediated Ca(2+) entry. Ca(2+) entry mediated by the TRP3 channel stably expressed in HEK293 cells was activated by phospholipase C-coupled receptors but independent of Ca(2+) store depletion (Ma, H.-T., Patterson, R. L., van Rossum, D. B., Birnbaumer, L., Mikoshiba, K., and Gill, D. L. (2000) Science 287, 1647-1651). Receptor-induced TRP3 activation was 2-APB-sensitive and fully blocked by MDL. Direct stimulation of TRP3 channels by the permeant diacylglycerol derivative, 1-oleoyl-2-acetyl-sn-glycerol, was not blocked by 2-APB, but was again prevented by MDL. The results indicate that although the activation and coupling processes for each of the three entry mechanisms are distinct, sensitivity to MDL is a feature shared by all three mechanisms, suggesting there may be a common structural feature in the channels themselves or an associated regulatory component.
RMI 12330A, an inhibitor of cyclic nucleotide phosphodiesterases and adenylate cyclase in kidney preparations.[Pubmed:6256001]
Biochim Biophys Acta. 1980 Jun 13;613(2):499-506.
N-(cis-2-phenylcyclopentyl)azacyclotridecan-2-imine hydrochloride (RMI 12330A) inhibited cyclic AMP and cyclic GMP phosphodiesterase activities in kidney preparations from rat and mouse. The drug was effective in the concentration range 0.1-1 mM. The agent was much less effective in inhibiting chick kidney cyclic nucleotide phosphodiesterases. The onset of inhibition of rat particulate cyclic AMP phosphodiesterase activities was rapid (less than 30 s) and irreversible. The inhibition of the low Km forms of cyclic AMP phosphodiesterase in mouse kidney homogenates was of the non-competitive type. RMI 12330A inhibited cyclic AMP phosphodiesterase activities in intact rat renal tubules. Adenylate cyclase activity, both basal and stimulated, was inhibited in all three species by the drug. Since RMI 12330A affects cyclic GMP metabolism as well as cyclic AMP metabolism, caution must be exercised in interpreting its effects upon cellular processes in terms of its actions upon the adenylate cyclase-cyclic AMP pathway alone.
RMI 12330 A, an inhibitor of adenylate cyclase in rat liver.[Pubmed:911855]
Biochim Biophys Acta. 1977 Oct 13;484(2):465-75.
RMI 12330 A, (N,-(cis-2-phenylcyclopentyl) azacyclotridecan-2-imine hydrochloride), has been reported to inhibit choleratoxin-induced intestinal hypersecretion, presumably via an inhibition of mucosal adenylate cyclase (ATP:pyrophosphate-lyase (cyclizing), EC 4.6.1.1). We report here that the adenylate cyclase activity of a rat liver plasma membrane preparation was inhibited by concentrations of RMI 12330 A ranging from 10 muM to 5mM. Similar effects were observed when the adenylate cyclase preparation was assayed in the presence of 10 mM NaF, 0.1 muM glucagon or 1 muM (--)-epinephrine plus 10 muM GTP. The effect of RMI 12330 A was not due to the inhibition of the regenerating system present in the incubation medium, since the effect was preserved in its absence. The inhibition brought about by RMI 12330 A was due to a decrease in the maximal velocity of the reaction; the affinity of the enzyme for the substrate remained unmodified. The inhibition was immediate and irreversible, even after several washes of the membranes previously preincubated with the drug. Complete inhibition of cyclase was obtained at a concentration of 370 nmol of RMI 12330 A per mg of membrane protein. The drug acted with a similar dose-response curve upon intact as well as detergent-dispersed cyclase preparations.


