Propranolol HClCAS# 318-98-9 |
- Pregnenolone
Catalog No.:BCN6255
CAS No.:145-13-1
- Adrenosterone
Catalog No.:BCC4061
CAS No.:382-45-6
- Epiandrosterone
Catalog No.:BCC4481
CAS No.:481-29-8
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 318-98-9 | SDF | Download SDF |
| PubChem ID | 62882 | Appearance | Powder |
| Formula | C16H22ClNO2 | M.Wt | 295.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
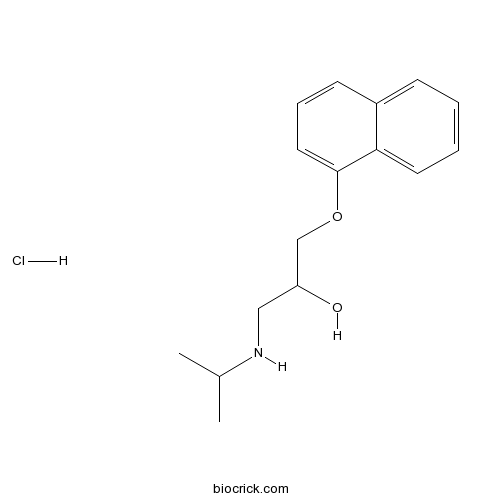
| Chemical Name | 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol;hydrochloride | ||
| SMILES | CC(C)NCC(COC1=CC=CC2=CC=CC=C21)O.Cl | ||
| Standard InChIKey | ZMRUPTIKESYGQW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H21NO2.ClH/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16;/h3-9,12,14,17-18H,10-11H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Propranolol hydrochloride is a nonselective β-adrenergic receptor (βAR) antagonist with an IC50 of 12 nM.In Vitro:In cultured endothelial or tumor cells, propranolol has been shown to both reduce cAMP levels and simultaneously activate the mitogen-activated protein kinase (MAPK) pathway downstream of βAR inhibition[2]. It displays high affinity for 5-HT1B receptors (Ki= 17 nM), and milder affinity for 5HT1D receptors (Ki= 10.2 μM)[3].In Vivo:Chronic administration of propranolol increased the beta(1)-adrenoceptors but decreased the beta(2)-adrenoceptors without changing total amount of plasma membrane beta-adrenoceptors[4]. References: | |||||

Propranolol HCl Dilution Calculator

Propranolol HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3807 mL | 16.9033 mL | 33.8066 mL | 67.6133 mL | 84.5166 mL |
| 5 mM | 0.6761 mL | 3.3807 mL | 6.7613 mL | 13.5227 mL | 16.9033 mL |
| 10 mM | 0.3381 mL | 1.6903 mL | 3.3807 mL | 6.7613 mL | 8.4517 mL |
| 50 mM | 0.0676 mL | 0.3381 mL | 0.6761 mL | 1.3523 mL | 1.6903 mL |
| 100 mM | 0.0338 mL | 0.169 mL | 0.3381 mL | 0.6761 mL | 0.8452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Propranolol Hydrochloride is a widely used non-cardioselective beta-adrenergic antagonist.
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
- Moricizine
Catalog No.:BCC5235
CAS No.:31883-05-3
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
- H-β-Ala-OMe.HCl
Catalog No.:BCC2853
CAS No.:3196-73-4
- Ethyl glucoside
Catalog No.:BCN5239
CAS No.:3198-49-0
Statistical optimization of a novel excipient (CMEC) based gastro retentive floating tablets of propranolol HCl and it's in vivo buoyancy characterization in healthy human volunteers.[Pubmed:23351981]
Daru. 2012 Aug 30;20(1):21.
The objective of the present investigation is to formulate gastro retentive floating drug delivery systems (GRFDDS) of Propranolol HCl by central composite design and to study the effect of formulation variables on floating lag time, D1hr (% drug release at 1 hr) and t90 (time required to release 90% of the drug). 3 factor central composite design was employed for the development of GRFDDS containing novel semi synthetic polymer carboxymethyl ethyl cellulose (CMEC) as a release retarding polymer. CMEC, sodium bicarbonate and Povidone concentrations were included as independent variables. The tablets were prepared by direct compression method and were evaluated for in vitro buoyancy and dissolution studies. From the polynomial model fitting statistical analysis, it was confirmed that the response floating lag time and D1hr is suggested to quadratic model and t90 is suggested to linear model. All the statistical formulations followed first order rate kinetics with non-Fickian diffusion mechanism. The desirability function was used to optimize the response variables, each having a different target, and the observed responses were highly agreed with experimental values. Statistically optimized formulation was characterized by FTIR and DSC studies and found no interactions between drug and polymer. The results demonstrate the feasibility of the model in the development of GRFDDS containing a Propranolol HCl. Statistically optimized formulation was evaluated for in vivo buoyancy studies in healthy humans for both fed and fasted states. From the results, it was concluded that gastric residence time of the floating tablets were enhanced at fed stage but not in fasted state.
Thermal sintering: a novel technique used in the design, optimization and biopharmaceutical evaluation of propranolol HCl gastric floating tablets.[Pubmed:23317339]
Drug Dev Ind Pharm. 2014 Jan;40(1):33-45.
The objective of the present investigation was to study the applicability of thermal sintering technique for the development of gastric floating tablets of Propranolol HCl. Formulations were prepared using four independent variables, namely (i) polymer quantity, (ii) sodium bicarbonate concentration, (iii) sintering temperature and (iv) sintering time. Floating lag time and t95 were taken as dependent variables. Tablets were prepared by the direct compression method and were evaluated for physicochemical properties, in vitro buoyancy and dissolution studies. From the drug release studies, it was observed that drug retarding property mainly depends upon the sintering temperature and time of exposure. The statistically optimized formulation (PTSso) was characterized by Fourier transform infrared spectroscopy and differential scanning calorimetry studies, and no significant chemical interaction between drug and polymer was observed. Optimized formulation was stable at accelerated conditions for a period of six months. PTSso was evaluated for in vivo buoyancy studies in humans for both fed and fasted states and found that gastric residence time of the floating tablets were enhanced by fed stage but not in fasted state. Optimized formulation PTSso and commercial formulation Ciplar LA 80 were subjected to bioavailability studies in healthy human volunteers by estimating pharmacokinetic parameters such as Cmax, Tmax, area under curve (AUC), elimination rate constant (Kel), biological half-life (t1/2) and mean residence time (MRT). There was a significant increase in the bioavailability of the Propranolol HCl from PTSso formulation, which was evident from increased AUC levels and larger MRT values than Ciplar LA 80.
Assessment of xanthan gum based sustained release matrix tablets containing highly water-soluble propranolol HCl.[Pubmed:23614284]
Acta Pol Pharm. 2013 Mar-Apr;70(2):283-9.
The present study was carried out to develop oral sustained release tablets of Propranolol HCl by different ratios of drug : matrix. Tablets were prepared by direct compression technique using xanthan gum and lactose. All the formulations (tablets) were evaluated for thickness, diameter, hardness, friability, weight variation, content of active ingredient, in vitro dissolution using USP dissolution apparatus-II and swelling index. In case of dissolution, an inverse relationship was noted between amount of xanthan gum and release rate of Propranolol HCl and the drug release was gradually enhanced as the amount of the lactose increased. The direct release was observed between swelling index and xanthan gum concentration. Significant difference in different media was observed in release profile, indicating that propranolol HCI has better solubility in HCI buffer pH 1.2. Moreover, dissolution data at differing stirring speeds was also analyzed, indicating that the drug release profile was at 50 rpm comparative to 100 rpm. The kinetic treatment showed the best fitted different mathematical models (zero order, first order, Higuchi's, Hixson-Crowell and Korsmeyer Peppas model. Most of the formulations showed linearity in Higuchi's model. The drug release from these tablets was by Fickian diffusion and anomalous (non-Fickian) mechanisms.
Chronotherapeutic drug delivery of Tamarind gum, Chitosan and Okra gum controlled release colon targeted directly compressed Propranolol HCl matrix tablets and in-vitro evaluation.[Pubmed:25936283]
Int J Biol Macromol. 2015 Aug;79:290-9.
The main objective of this investigation is to develop a chronotherapeutic drug delivery of various natural polymers based colon targeted drug delivery systems to treat early morning sign in BP. The polymers such as Tamarind gum, Okra gum and Chitosan were used in the formulation design. A model drug Propranolol HCl was incorporated in the formulation in order to assess the controlled release and time dependent release potential of various natural polymers. A novel polymer Tamarind gum was extracted and used as a prime polymer in this study to prove the superiority of this polymer over other leading natural polymer. Propranolol HCl was used as a model drug which undergoes hepatic metabolism and witnesses the poor bioavailability. The matrix tablets of Propranolol HCl were prepared by direct compression. The tablets were evaluated for various quality control parameters and found to be within the limits. Carbopol 940 was used as an auxiliary polymer to modify the drug release and physicochemical characteristics of the tablets. The in vitro release studies were performed in 0.1N HCl for 1.5h, followed by pH 6.8 phosphate buffer for 2h and pH 7.4 phosphate buffer till maximum amount of drug release. The in vitro release profile of the formulations were fitted with various pharmacokinetic mathematical models and analyzed for release profile. The formulations prepared with Tamarind gum prolonged the release for an extended period of time compared to other polymer based formulation and showed an excellent compression characteristic.


