Methyl orsellinateCAS# 3187-58-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3187-58-4 | SDF | Download SDF |
| PubChem ID | 76658 | Appearance | Powder |
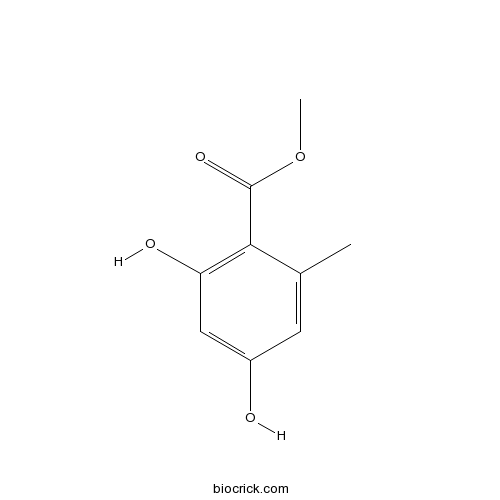
| Formula | C9H10O4 | M.Wt | 182.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl 2,4-dihydroxy-6-methylbenzoate | ||
| SMILES | CC1=CC(=CC(=C1C(=O)OC)O)O | ||
| Standard InChIKey | NCCWCZLEACWJIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H10O4/c1-5-3-6(10)4-7(11)8(5)9(12)13-2/h3-4,10-11H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Methyl orsellinate is a phytotoxic compound, it can cause significant inhibition of radicle growth of Amaranthus hypochondriacus and Echinochloa crus-galli, interact with bovine-brain calmodulin and inhibit the activation of the calmodulin-dependent enzyme cAMP phosphodiesterase. 2. Methyl orsellinate exhibits antifungal activity. 3. Methyl orsellinate can inhibit PTP1B activity with 50% inhibitory concentration values of 277 +/- 8.6 microM, the selective inhibition of PTP1B has been widely recognized as a potential drug target for the treatment of type 2 diabetes and obesity, methyl orsellinate may can treat the type 2 diabetes and obesity. |
| Targets | cAMP | Antifection |

Methyl orsellinate Dilution Calculator

Methyl orsellinate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4885 mL | 27.4424 mL | 54.8847 mL | 109.7695 mL | 137.2119 mL |
| 5 mM | 1.0977 mL | 5.4885 mL | 10.9769 mL | 21.9539 mL | 27.4424 mL |
| 10 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 50 mM | 0.1098 mL | 0.5488 mL | 1.0977 mL | 2.1954 mL | 2.7442 mL |
| 100 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- Propranolol HCl
Catalog No.:BCC4336
CAS No.:318-98-9
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- Moricizine
Catalog No.:BCC5235
CAS No.:31883-05-3
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
- H-β-Ala-OMe.HCl
Catalog No.:BCC2853
CAS No.:3196-73-4
- Ethyl glucoside
Catalog No.:BCN5239
CAS No.:3198-49-0
- SIB 1757
Catalog No.:BCC6971
CAS No.:31993-01-8
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Aclidinium Bromide
Catalog No.:BCC4575
CAS No.:320345-99-1
- Marmin acetonide
Catalog No.:BCN5240
CAS No.:320624-68-8
Effects of tenuiorin and methyl orsellinate from the lichen Peltigera leucophlebia on 5-/15-lipoxygenases and proliferation of malignant cell lines in vitro.[Pubmed:12487331]
Phytomedicine. 2002 Oct;9(7):654-8.
The orcinol derivatives tenuiorin (1) and Methyl orsellinate (2) were identified as active components of an extract from the lichen Peltigera leucophlebia (Nyl.) Gyeln. showing in vitro inhibitory activity against 15-lipoxygenase from soybeans. The compounds were subsequently tested for in vitro activity against 5-lipoxygenase from porcine leucocytes and proved to be moderately active, with IC50 values of 41.6 microM and 59.6 microM respectively. Tenuiorin is a known constituent of several Peltigera species but has not previously been isolated from P. leucophlebia. As correlation between 5-lipoxygenase inhibition and antiproliferative effects has earlier been witnessed for related lichen metabolites, tenuiorin and Methyl orsellinate were further tested for antiproliferative activity on cultured human breast (T-47D)-, pancreatic (PANC-1)- and colon (WIDR) cancer cell lines. The monomeric Methyl orsellinate exhibited no detectable antiproliferative activity whereas the trimeric tenuiorin caused moderate/weak reduction in [3H]-thymidine uptake of the pancreatic- and colon cancer cells, with ED50 values of 87.9 and 98.3 microM respectively.
Phytotoxic compounds from Flourensia cernua.[Pubmed:12946427]
Phytochemistry. 2003 Sep;64(1):285-91.
Bioassay-directed fractionation of a CH(2)Cl(2)-MeOH (1:1) extract of the aerial parts of Flourensia cernua led to the isolation of three phytotoxic compounds, namely, dehydroflourensic acid (1), flourensadiol (2) and Methyl orsellinate (3). Dehydroflourensic acid is a new natural product whose structure was established by spectral means. In addition, the known flavonoid ermanin and seven hitherto unknown gamma-lactones were obtained, these being tetracosan-4-olide, pentacosan-4-olide, hexacosan-4-olide, heptacosan-4-olide, octacosan-4-olide, nonacosan-4-olide, and triacontan-4-olide. Compounds 1-3 caused significant inhibition of radicle growth of Amaranthus hypochondriacus and Echinochloa crus-galli, interacted with bovine-brain calmodulin and inhibited the activation of the calmodulin-dependent enzyme cAMP phosphodiesterase.
PTP1B inhibitory effects of tridepside and related metabolites isolated from the Antarctic lichen Umbilicaria antarctica.[Pubmed:19619069]
J Enzyme Inhib Med Chem. 2009 Oct;24(5):1133-7.
The selective inhibition of PTP1B has been widely recognized as a potential drug target for the treatment of type 2 diabetes and obesity. In the course of screening for PTP1B inhibitory natural products, the MeOH extract of the dried sample of the Antarctic lichen Umbilicaria antarctica was found to exhibit significant inhibitory effect, and the bioassay-guided fractionation and purification afforded three related lichen metabolites 1-3. Compounds 1-3 were identified as gyrophoric acid (1), lecanoric acid (2), and Methyl orsellinate (3) mainly by analysis of NMR and MS data. These compounds inhibited PTP1B activity with 50% inhibitory concentration values of 3.6 +/- 0.04 microM, 31 +/- 2.7 microM, and 277 +/- 8.6 microM, respectively. Furthermore, the kinetic analysis of PTP1B inhibition by compound 1 suggested that the compound inhibited PTP1B activity in a non-competitive manner.


