(-)-LyoniresinolCAS# 31768-94-2 |
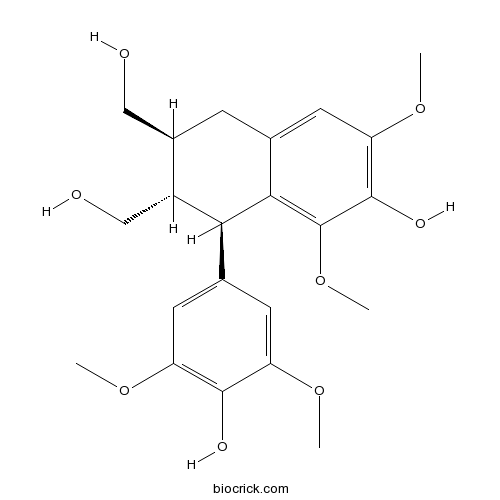
- (+)-Lyoniresinol
Catalog No.:BCN6248
CAS No.:14464-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31768-94-2 | SDF | Download SDF |
| PubChem ID | 9888378 | Appearance | Cryst. |
| Formula | C22H28O8 | M.Wt | 420.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6S,7S,8R)-8-(4-hydroxy-3,5-dimethoxyphenyl)-6,7-bis(hydroxymethyl)-1,3-dimethoxy-5,6,7,8-tetrahydronaphthalen-2-ol | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C(C(CC3=CC(=C(C(=C23)OC)O)OC)CO)CO | ||
| Standard InChIKey | ZDVZKBOFCHOPLM-LBTNJELSSA-N | ||
| Standard InChI | InChI=1S/C22H28O8/c1-27-15-7-12(8-16(28-2)20(15)25)18-14(10-24)13(9-23)5-11-6-17(29-3)21(26)22(30-4)19(11)18/h6-8,13-14,18,23-26H,5,9-10H2,1-4H3/t13-,14-,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Lyoniresinol has antioxidant activity. |

(-)-Lyoniresinol Dilution Calculator

(-)-Lyoniresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3781 mL | 11.8906 mL | 23.7812 mL | 47.5624 mL | 59.453 mL |
| 5 mM | 0.4756 mL | 2.3781 mL | 4.7562 mL | 9.5125 mL | 11.8906 mL |
| 10 mM | 0.2378 mL | 1.1891 mL | 2.3781 mL | 4.7562 mL | 5.9453 mL |
| 50 mM | 0.0476 mL | 0.2378 mL | 0.4756 mL | 0.9512 mL | 1.1891 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2378 mL | 0.4756 mL | 0.5945 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- GW501516
Catalog No.:BCC2268
CAS No.:317318-70-0
- 3,5,7-Trihydroxychromone
Catalog No.:BCN7479
CAS No.:31721-95-6
- 5,7-Dihydroxychromone
Catalog No.:BCN4652
CAS No.:31721-94-5
- Hesperetin 7-O-glucoside
Catalog No.:BCN5237
CAS No.:31712-49-9
- Aminophylline
Catalog No.:BCC2300
CAS No.:317-34-0
- Levomefolic acid
Catalog No.:BCC1703
CAS No.:31690-09-2
- Pinusolide
Catalog No.:BCN5236
CAS No.:31685-80-0
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- Propranolol HCl
Catalog No.:BCC4336
CAS No.:318-98-9
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
- Moricizine
Catalog No.:BCC5235
CAS No.:31883-05-3
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
Development of a quantitation method to assay both lyoniresinol enantiomers in wines, spirits, and oak wood by liquid chromatography-high resolution mass spectrometry.[Pubmed:27000563]
Anal Bioanal Chem. 2016 May;408(14):3789-99.
Wine taste balance evolves during oak aging by the release of volatile and non-volatile compounds from wood. Among them, an enantiomer of lyoniresinol, (+)-lyoniresinol, has been shown to exhibit bitterness. To evaluate the impact of (+)-lyoniresinol on wine taste, a two-step quantitation method was developed and validated. First, (+/-)-lyoniresinol was assayed in wines, spirits, and oak wood macerates by C-18 liquid chromatography-high resolution mass spectrometry (LC-HRMS). Then, the lyoniresinol enantiomeric ratio was determined by chiral LC-HRMS in order to calculate the (+)-lyoniresinol content. In red and white wines, the average concentrations of (+)-lyoniresinol were 1.9 and 0.8 mg/L, respectively. The enantiomer proportions were not affected by bottle aging, and lyoniresinol appeared to remain stable over time. The sensory study of (+)-lyoniresinol established its perception threshold at 0.46 mg/L in wine. All the commercial wines quantitated were above this perception threshold, demonstrating its impact on wine taste by an increase in bitterness. In spirits, (+)-lyoniresinol ranged from 2.0 to 10.0 mg/L and was found to be released continuously during oak aging. Finally, neither botanical origin nor toasting was found to significantly affect the (+)-lyoniresinol content of oak wood. Graphical abstract From oak wood to wine: evaluation of the influence of (+)-lyoniresinol on the bitterness of wines and spirits.
Enhanced anti-oxidative effect of fermented Korean mistletoe is originated from an increase in the contents of caffeic acid and lyoniresinol.[Pubmed:27072079]
Food Funct. 2016 May 18;7(5):2270-7.
Viscum album var. coloratum (Korean mistletoe; KM) is an herbal medicine that is used worldwide for the treatment of various immunological disorders and cancers. KM extract showed enhanced anti-oxidative effects in 2,2-diphenyl-1-picrylhydrazyl, Trolox equivalent antioxidant capacity, and 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate acetyl ester assays after being fermented with a crude enzyme extract from a soybean paste fungus, Aspergillus kawachii. High-performance liquid chromatography analysis showed four increased peaks in enzyme treated KM. The increased peaks were isolated and identified as caffeic acid (1), hesperetin (2), syringaldehyde (3), and lyoniresinol (4). Among the four compounds, only 1 and 4 showed strong anti-oxidative activity. Therefore, the fermentation increased the contents of 1 and 4, which consequently increased the anti-oxidative activity of KM.
Lyoniresinol 3alpha-O-beta-D-glucopyranoside-mediated hypoglycaemia and its influence on apoptosis-regulatory protein expression in the injured kidneys of streptozotocin-induced mice.[Pubmed:24312585]
PLoS One. 2013 Dec 3;8(12):e81772.
Averrhoa carambola L. (Oxalidaceae) root (ACLR) has a long history of use in traditional Chinese medicine for treating diabetes and diabetic nephropathy (DN). (+/-)-Lyoniresinol 3alpha-O-beta-D-glucopyranoside (LGP1, LGP2) were two chiral lignan glucosides that were isolated from the ACLR. The purpose of this study was to investigate the effect of LGP1 and LGP2-mediated hypoglycaemia on renal injury in streptozotocin (STZ)-induced diabetic mice. STZ-induced diabetic mice were administrated LGP1 and LGP2 orally (20, 40, 80 mg/kg body weight/d) for 14 days. Hyperglycaemia and the expression of related proteins such as nuclear factor-kappaB (NF-kappaB), caspase-3, -8, -9, and Bcl-associated X protein (Bax) were markedly decreased by LGP1 treatment. However, LGP2 treatment had no hypoglycaemic activity. Diabetes-dependent alterations in the kidney such as glomerular hypertrophy, excessive extracellular matrix amassing, and glomerular and tubular basement membrane thickening were improved after 14 days of LGP1 treatment. B cell lymphoma Leukaemia-2 (Bcl-2) expression was reduced in the STZ-induced diabetic mouse kidneys but was enhanced by LGP1 treatment. These findings suggest that LGP1 treatment may inhibit diabetic nephropathy progression and may regulate several pharmacological targets for treating or preventing diabetic nephropathy.
A new lyoniresinol derivative from Smilax microphylla.[Pubmed:23472472]
Nat Prod Commun. 2013 Jan;8(1):113-4.
A new lignan, lyoniresinol-9-O-8"-syringylglycerol ether (1), together with five known compounds, piceatannol (2), resveratrol (3), oxyresveratrol (4), quercetin-3'-glucoside (5) and diosgenin (6) were isolated from the rhizomes of Smilax microphylla. The structure of the new compound was determined by means of chemical evidence and 1D-and 2D-NMR (1H, 13C, HSQC, HMBC, 1H-1H COSY and NOESY) spectroscopic analysis and HR-ESI-MS.
How stereochemistry influences the taste of wine: Isolation, characterization and sensory evaluation of lyoniresinol stereoisomers.[Pubmed:26320975]
Anal Chim Acta. 2015 Aug 12;888:191-8.
Wine expresses its beauty by sending a sensory message to the taster through molecules coming from grapes, yeast metabolism or oak wood. Among the compounds released during barrel aging, lyoniresinol has been recently reported as a relevant contributor to wine bitterness. As this lignan contains three stereogenic carbons, this work aimed at investigating the influence of stereochemistry on wine taste by combining analytical and sensorial techniques. First, an oak wood extract was screened by Liquid Chromatography-High Resolution Mass Spectrometry to target isomers separable in a symmetric environment and a diastereoisomer called epi-lyoniresinol was isolated for the first time. Then, an original racemic resolution based on natural xylose-derivatives was carried out to obtain lyoniresinol enantiomers. Chiroptical spectroscopic measurements associated with theoretical calculations allowed the unambiguous determination of their absolute configuration. The taste properties of all these stereoisomers revealed that only one lyoniresinol enantiomer is strongly bitter whereas the other one is tasteless and the diastereoisomer is slightly sweet. The presence of these three compounds was established in an oaked Bordeaux wine by chiral and non-chiral chromatography, suggesting the significant influence of stereochemistry on wine taste.


