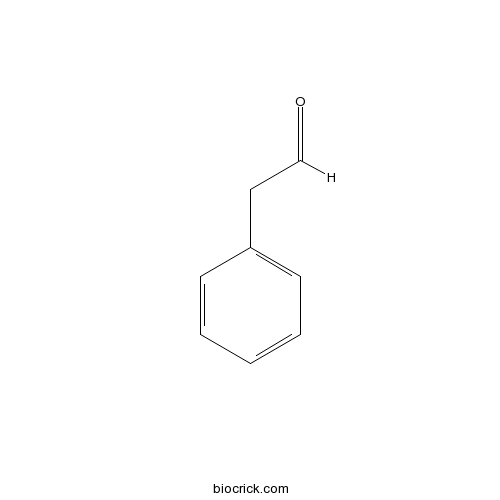
PhenylacetaldehydeCAS# 122-78-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122-78-1 | SDF | Download SDF |
| PubChem ID | 998 | Appearance | Powder |
| Formula | C8H8O | M.Wt | 120.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-phenylacetaldehyde | ||
| SMILES | C1=CC=C(C=C1)CC=O | ||
| Standard InChIKey | DTUQWGWMVIHBKE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O/c9-7-6-8-4-2-1-3-5-8/h1-5,7H,6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phenylacetaldehyde is a oviposition inhibitor for the Pink Bollworm, it can attract Moths to bladder flower and to blacklight traps. Phenylacetaldehyde and carbon monoxide as effective additives for the selective hydrogenation of cyclooctadienes to cyclooctene over palladium catalysts. |
| Targets | Oviposition inhibitor |
| In vitro | Phenylacetaldehyde Attracts Moths to Bladder Flower and to Blacklight Traps[Reference: WebLink]Environmental Entomology, 1979 ,8 (3) :444-7.The fragrant chemical compound volatilized by the bladder flower, Araujia sericofera Brot., was identified as Phenylacetaldehyde. It attracted many economically important species of moths, and when it was used with a blacklight trap, it increased trap collections of pest moth species by 48%. |
| In vivo | Phenylacetaldehyde: Oviposition Inhibitor for the Pink Bollworm[Reference: WebLink]Journal of Economic Entomology , 1977 , 70 (5) :547-8.Field plots of cotton treated with methyl salicylate or untreated had significantly greater infestations of Pectinophora gossypiella (Saunders) than similar plots treated with Phenylacetaldehyde. |
| Structure Identification | Bulletin of the Chemical Society of Japan, 2006 , 65 (11) :2960-5.Phenylacetaldehyde and Carbon Monoxide as Effective Additives for the Selective Hydrogenation of Cyclooctadienes to Cyclooctene over Palladium Catalysts[Reference: WebLink]
|

Phenylacetaldehyde Dilution Calculator

Phenylacetaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.3195 mL | 41.5973 mL | 83.1947 mL | 166.3894 mL | 207.9867 mL |
| 5 mM | 1.6639 mL | 8.3195 mL | 16.6389 mL | 33.2779 mL | 41.5973 mL |
| 10 mM | 0.8319 mL | 4.1597 mL | 8.3195 mL | 16.6389 mL | 20.7987 mL |
| 50 mM | 0.1664 mL | 0.8319 mL | 1.6639 mL | 3.3278 mL | 4.1597 mL |
| 100 mM | 0.0832 mL | 0.416 mL | 0.8319 mL | 1.6639 mL | 2.0799 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cinnamyl cinnamate
Catalog No.:BCN4722
CAS No.:122-69-0
- (-)-Ampelopsin H
Catalog No.:BCC8842
CAS No.:
- Zingerone
Catalog No.:BCN1192
CAS No.:122-48-5
- Glycerine trioleate
Catalog No.:BCN2287
CAS No.:122-32-7
- Tetraethoxypropane
Catalog No.:BCN2221
CAS No.:122-31-6
- Sulfadimethoxine
Catalog No.:BCC5159
CAS No.:122-11-2
- (±)-Anatoxin A fumarate
Catalog No.:BCC6796
CAS No.:1219922-30-1
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- PKA inhibitor fragment (6-22) amide
Catalog No.:BCC1042
CAS No.:121932-06-7
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
- Sophoraflavanone C
Catalog No.:BCN3543
CAS No.:121927-91-1
- 3-Phenyl-1-propanol
Catalog No.:BCC8102
CAS No.:122-97-4
- Sulfamonomethoxine
Catalog No.:BCC9156
CAS No.:1220-83-3
- Niazirin
Catalog No.:BCN7300
CAS No.:122001-32-5
- Cyhalofop
Catalog No.:BCC5474
CAS No.:122008-78-0
- PLP (139-151)
Catalog No.:BCC5920
CAS No.:122018-58-0
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
- Khayalenoid E
Catalog No.:BCN6111
CAS No.:1220508-29-1
- [bAla8]-Neurokinin A(4-10)
Catalog No.:BCC7137
CAS No.:122063-01-8
- 2'-O-Acetylsprengerinin C
Catalog No.:BCN6655
CAS No.:1220707-33-4
- Charantadiol A
Catalog No.:BCN3483
CAS No.:1220890-23-2
- 3,4-Dihydro-3,4-dihydroxynaphthalen-1(2H)-one
Catalog No.:BCN1602
CAS No.:1220891-22-4
- Auraptenol
Catalog No.:BCN6113
CAS No.:1221-43-8
Current Advances on Structure-Function Relationships of Pyridoxal 5'-Phosphate-Dependent Enzymes.[Pubmed:30891451]
Front Mol Biosci. 2019 Mar 5;6:4.
Pyridoxal 5'-phosphate (PLP) functions as a coenzyme in many enzymatic processes, including decarboxylation, deamination, transamination, racemization, and others. Enzymes, requiring PLP, are commonly termed PLP-dependent enzymes, and they are widely involved in crucial cellular metabolic pathways in most of (if not all) living organisms. The chemical mechanisms for PLP-mediated reactions have been well elaborated and accepted with an emphasis on the pure chemical steps, but how the chemical steps are processed by enzymes, especially by functions of active site residues, are not fully elucidated. Furthermore, the specific mechanism of an enzyme in relation to the one for a similar class of enzymes seems scarcely described or discussed. This discussion aims to link the specific mechanism described for the individual enzyme to the same types of enzymes from different species with aminotransferases, decarboxylases, racemase, aldolase, cystathionine beta-synthase, aromatic Phenylacetaldehyde synthase, et al. as models. The structural factors that contribute to the reaction mechanisms, particularly active site residues critical for dictating the reaction specificity, are summarized in this review.
A green triple-locked strategy based on volatile-compound imaging, chemometrics, and markers to discriminate winter honey and sapium honey using headspace gas chromatography-ion mobility spectrometry.[Pubmed:30884736]
Food Res Int. 2019 May;119:960-967.
A simple and environmentally approach using untargeted imaging of volatile substances combined with chemometrics and markers response was proposed for discriminating different species of honey with headspace gas-chromatography-ion-mobility (HS-GC-IMS). The 3D HS-GC-IMS imaging and their response differences enabled the clear discrimination between winter honey and sapium honey. Principal component analysis (PCA) and partial least-squares discrimination analysis (PLS-DA) were employed to discriminate different honey. Markers of two kinds of honey were identified and confirmed with a user-built imaging database combined with multivariate analysis. Benzaldehyde dimer and Phenylacetaldehyde dimer were found to be reliable markers of winter honey, and phenylethyl acetate dimer was of sapium honey. Adulteration identification of the honey samples with different adulteration ratios were subjected to this triple-locked strategy analysis. The results demonstrate that HS-GC-IMS imaging coupled with chemometrics and marker identification is a useful triple-locked strategy to discriminate honey from different floral origins and adulterated honey.
Characterization and differentiation of key odor-active compounds of 'Beibinghong' icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution.[Pubmed:30857688]
Food Chem. 2019 Jul 30;287:186-196.
Freezing-thawing events contribute to the unique aroma profile of icewines. Differences in key odor-active volatile compounds between 'Beibinghong' (Vitis amurensisxV. vinifera) icewines and dry wines were investigated by gas chromatography-olfactometry and gas chromatography-mass spectrometry. Acceptable agreement between the olfactometric and quantitative results was obtained. 'Beibinghong' icewine was characterized by high concentrations of volatile phenols, lactones, (E)-beta-damascenone, and Phenylacetaldehyde, which were associated with on-vine freezing-thawing events in grape. Low concentrations of higher alcohol acetates and ethyl esters of fatty acids were attributed to hyperosmotic stress during fermentation. The overall aroma of icewine could be mimicked by reconstitution containing 44 identified volatiles. Partial least squares regression analysis demonstrated that the concentrations of these volatile compounds determined the distinct sensory profiles of icewines, which have higher intensities of honey/sweet, smoky, caramel, dried fruit, apricot/peach, and floral aromas, and lower intensities of fresh fruity and herbaceous notes in comparison with dry wines.
Separate pathways contribute to the herbivore-induced formation of 2-phenylethanol in poplar.[Pubmed:30846485]
Plant Physiol. 2019 Mar 7. pii: pp.19.00059.
Upon herbivory, the tree species western balsam poplar (Populus trichocarpa) produces a variety of phenylalanine-derived metabolites, including 2-phenylethylamine, 2-phenylethanol, and 2-phenylethyl-beta-D-glucopyranoside. To investigate the formation of these potential defense compounds, we functionally characterized aromatic L-amino acid decarboxylases (AADCs) and aromatic aldehyde synthases (AASs), which play important roles in the biosynthesis of specialized aromatic metabolites in other plants. Heterologous expression in Escherichia coli and Nicotiana benthamiana showed that all five AADC/AAS genes identified in the P. trichocarpa genome encode active enzymes. However, only two genes, PtAADC1 and PtAAS1, were significantly upregulated after leaf herbivory. Despite a sequence similarity of about 96%, PtAADC1 and PtAAS1 showed different enzymatic functions and converted phenylalanine into 2-phenylethylamine and 2-Phenylacetaldehyde, respectively. The activities of both enzymes were interconvertible by switching a single amino acid residue in their active sites. A survey of putative AADC/AAS gene pairs in the genomes of other plants suggests an independent evolution of this function-determining residue in different plant families. RNAi-mediated downregulation of AADC1 in grey poplar (P. x canescens) resulted in decreased accumulation of 2-phenylethylamine and 2-phenylethyl-beta-D-glucopyranoside, while the emission of 2-phenylethanol was not influenced. To investigate the last step of 2-phenylethanol formation, we identified and characterized two P. trichocarpa short-chain dehydrogenases, PtPAR1 and PtPAR2, which were able to reduce 2-Phenylacetaldehyde to 2-phenylethanol in vitro. In summary, 2-phenylethanol and its glucoside may be formed in multiple ways in poplar. Our data indicate that PtAADC1 controls the herbivore-induced formation of 2-phenylethylamine and 2-phenylethyl-beta-D-glucopyranoside in planta, while PtAAS1 likely contributes to the herbivore-induced emission of 2-phenylethanol.
Aroma Patterns Characterization of Braised Pork Obtained from a Novel Ingredient by Sensory-Guided Analysis and Gas-Chromatography-Olfactometry.[Pubmed:30832317]
Foods. 2019 Mar 2;8(3). pii: foods8030087.
Two types of braised pork were prepared from self-made braised sauce added to Maillard reaction intermediate (MRI) and white granulated sugar, respectively. Descriptive sensory analysis and gas chromatography-mass spectrometry (GC-MS) were conducted to investigate their differences in sensory and aroma compounds. The results showed that the effect of self-made braised sauce in braised pork was comparable to white granulated sugar. One-hundred-and-nine volatile flavor compounds were identified by GC-MS using headspace-solid phase microextraction (HS-SPME) and simultaneous distillation and extraction (SDE). Thirty-six odor active compounds with retention indexes ranging from 935(-)2465 were identified by aroma extract dilution analysis (AEDA). Additionally, their odor activity values (OAV) were calculated. It was found that 17 aroma compounds showed an OAV greater than 1. Among them, pentanal (almond, pungent), nonanal (fat, green), (E, E)-2,4-decadienal (fat, roast), Phenylacetaldehyde (hawthorn, honey, sweet), dodecanal (lily, fat, citrus) and linalool (floral, lavender) reached the highest OAV values (>200), indicating a significant contribution to the aroma of two types of braised pork. These results indicated that the self-made braised sauce added with MRI could be used for cooking braised pork with good sensory characteristics.
CYP79D73 participates in biosynthesis of floral scent compound 2-phenylethanol in Plumeria rubra.[Pubmed:30804010]
Plant Physiol. 2019 Feb 25. pii: pp.19.00098.
Plumeria (Plumeria rubra), well-known for its brightly colored and fragrant flowers, emits a number of floral volatile organic compounds (VOCs). Plumeria flowers emit a total of 43 VOCs including nine phenylpropanoids/benzenoids, such as 2-phenylethanol (2PE), benzaldehyde, 2-Phenylacetaldehyde (PAld), (E/Z)-phenylacetaldoxime (PAOx), benzyl nitrile (BN), and 2-phenylnitroethane (PN). To identify genes and pathways involved in the production of the major compound 2PE, we analyzed the plumeria floral transcriptome and found a highly expressed, flower-specific gene encoding a cytochrome P450 (CYP450) family 79D protein (PrCYP79D73), which catalyzed the formation of (E/Z)-PAOx. Feeding experiments with deuterated phenylalanine (d5-Phe) or d5-(E/Z)-PAOx showed that (E/Z)-PAOx is an intermediate in the biosynthesis of 2PE, as are two nitrogen (N)-containing volatiles, BN and PN, in plumeria flowers. Crude enzyme extracts from plumeria flowers converted L-Phe to (E/Z)-PAOx, PAld, 2PE, BN, and PN. The biosynthesis of these compounds increased with addition of PrCYP79D73-enriched microsomes but was blocked by pretreatment with 4-phenylimidazole, an inhibitor of CYP450 enzymes. Moreover, overexpression of PrCYP79D73 in Nicotiana benthamiana resulted in the emission of (E/Z)-PAOx as well as PAld, 2PE, BN, and PN, all of which were also found among plumeria floral VOCs. Taken together, our results demonstrate that PrCYP79D73 is a crucial player in the biosynthesis of the major floral VOC 2PE and other N-containing volatiles. These volatiles may be required for plant defense as well as to attract pollinators for the successful reproduction of plumeria.
Alcohol dehydrogenases from Proteus mirabilis contribute to alcoholic flavor.[Pubmed:30761541]
J Sci Food Agric. 2019 Feb 13.
BACKGROUND: Cheese ripening involves a complex series of metabolic reactions and numerous concomitant secondary transformations. Alcohol dehydrogenase (ADH) converts aldehydes into their corresponding alcohols, which enrich cheese aroma. RESULTS: In this study, we identified five ADH genes in Proteus mirabilis JN458, and these genes were overexpressed and characterized in Escherichia coli BL21 (DE3). The optimum pH was 7.0 for the purified recombinant ADH-1, ADH-2, and ADH-3 and 8.0 for ADH-4 and ADH-5. The optimum temperature was 40 degrees C for ADH-1, ADH-3, and ADH-5 and 45 degrees C for ADH-2 and ADH-4. The Km value of ADH-1, ADH-2, and ADH-3 was 34.45, 16.90, and 10.01 micromol L(-1) for Phenylacetaldehyde, respectively. The Km value of ADH-4 and ADH-5 was 14.81 and 24.62 micromol L(-1) for 2-methylbutanal, respectively. CONCLUSION: Proteus species play important roles during cheese ripening. The results of our study are important for further research on cheese flavor and for quality control during cheese production. (c) 2019 Society of Chemical Industry.
Assessment of volatile compounds and sensory characteristics of Mexican hibiscus (Hibiscus sabdariffa L.) calyces hot beverages.[Pubmed:30728578]
J Food Sci Technol. 2019 Jan;56(1):360-366.
This study was conducted to assess and correlate the sensory characteristics and volatile compounds of hot beverages from the calyces of four Mexican varieties of hibiscus (4Q4, Puebla Precoz, UAN 16-1, and Sudan). A panel of 10 judges, detected six flavour descriptors in all samples. Sensory studies revealed highly characteristic flavour profiles of these varieties. In order to obtain the extracts and further characterize the odour-active volatiles of the studied beverages, a simultaneous steam distillation and solvent extraction procedure followed by a GC-MS analysis was employed. A total of 104 volatile compounds were identified in all samples. By determining the odour activity values (OAVs) it was possible to identify compounds with high odor-activity in the beverages, such as: 2-furfural, 5-methyl-2-furfural, hexanal, (E)-2-hexenal, (Z)-3-hexen-1-ol, 1-octen-3-one, 1-octen-3-ol, 5-methyl-2(3H)-furanone, Phenylacetaldehyde, nonanal, (E)-2-nonenal, geranylacetone, alpha-ionone and beta-ionone. Moreover, on the basis of their OAVs, the differences in odour profiles of beverages were predominately due to these odorants.
Response surface methodology: A tool to minimize aldehydes formation and oxygen consumption in wine model system.[Pubmed:30722912]
Food Chem. 2019 Jun 15;283:559-565.
A response surface methodology was applied to study the effect of precursors on o-quinone and Phenylacetaldehyde formation in wine model systems stored at 40 degrees C during 24h. The results confirmed that glucose plays an important role in reducing aldehyde formation by inhibiting the formation of o-quinone. The regression equations showed that oxygen consumption followed a 2nd polynomial equation whereas Phenylacetaldehyde and o-quinone were best fit with a polynomial function containing quadratic terms. These behaviors indicate that different pathways are involved in the respective aldehyde formation and oxygen consumption. RSM has been shown to be a powerful tool to better understand key chemical reactions. By considering a number of factors, individually and in combinations, the derived equations predicted that the best combination to minimize Phenylacetaldehyde was achieved for high glucose levels and low amounts of gallic acid and metals. This is valuable information when trying to improve wines sensorial properties during shelf-life.
Metabolic Engineering of Escherichia coli for para-Amino-Phenylethanol and para-Amino-Phenylacetic Acid Biosynthesis.[Pubmed:30662895]
Front Bioeng Biotechnol. 2019 Jan 4;6:201.
Aromatic amines are an important class of chemicals which are used as building blocks for the synthesis of polymers and pharmaceuticals. In this study we establish a de novo pathway for the biosynthesis of the aromatic amines para-amino-phenylethanol (PAPE) and para-amino-phenylacetic acid (4-APA) in Escherichia coli. We combined a synthetic para-amino-l-phenylalanine pathway with the fungal Ehrlich pathway. Therefore, we overexpressed the heterologous genes encoding 4-amino-4-deoxychorismate synthase (pabAB from Corynebacterium glutamicum), 4-amino-4-deoxychorismate mutase and 4-amino-4-deoxyprephenate dehydrogenase (papB and papC from Streptomyces venezuelae) and ThDP-dependent keto-acid decarboxylase (aro10 from Saccharomyces cerevisiae) in E. coli. The resulting para-amino-Phenylacetaldehyde either was reduced to PAPE or oxidized to 4-APA. The wild type strain E. coli LJ110 with a plasmid carrying these four genes produced (in shake flask cultures) 11 +/- 1.5 mg l(-1) of PAPE from glucose (4.5 g l(-1)). By the additional cloning and expression of feaB (Phenylacetaldehyde dehydrogenase from E. coli) 36 +/- 5 mg l(-1) of 4-APA were obtained from 4.5 g l(-1) glucose. Competing reactions, such as the genes for aminotransferases (aspC and tyrB) or for biosynthesis of L-phenylalanine and L-tyrosine (pheA, tyrA) and for the regulator TyrR were removed. Additionally, the E. coli genes aroFBL were cloned and expressed from a second plasmid. The best producer strains of E. coli showed improved formation of PAPE and 4-APA, respectively. Plasmid-borne expression of an aldehyde reductase (yahK from E. coli) gave best values for PAPE production, whereas feaB-overexpression led to best values for 4-APA. In fed-batch cultivation, the best producer strains achieved 2.5 +/- 0.15 g l(-1) of PAPE from glucose (11% C mol mol-1 glucose) and 3.4 +/- 0.3 g l(-1) of 4-APA (17% C mol mol(-1) glucose), respectively which are the highest values for recombinant strains reported so far.
Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees.[Pubmed:30658764]
Food Chem. 2019 May 30;281:49-56.
This work presents a headspace-solid phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) method for the analysis of solid food samples in extended experiments. The final procedure was used to quantify 30 volatile compounds in fresh beef. The strategy adds robustness to the classic SPME methods for solid samples, by including a control solution that solves several challenges. The control solution contained one representative compound for each studied family of beef, and two internal standards. Response factors were calculated for each family, and were subsequently applied to every compound belonging to the same family. This strategy allowed control of the quantification procedure even when the fibre, column or control solution changed. Repeatability and reproducibility had relative standard deviation values below 17%, except for Phenylacetaldehyde, (E)-2-nonenal and (E,Z)-2,4-decadienal. Although the method described here was applied to animal products, it has also been successfully used to distinguish between samples from different lipid oxidation stabilities.
Biosynthesis of 2-Phenylethanol in Rose Petals Is Linked to the Expression of One Allele of RhPAAS.[Pubmed:30622153]
Plant Physiol. 2019 Mar;179(3):1064-1079.
Floral scent is one of the most important characters in horticultural plants. Roses (Rosa spp.) have been cultivated for their scent since antiquity. However, probably by selecting for cultivars with long vase life, breeders have lost the fragrant character in many modern roses, especially the ones bred for the cut flower market. The genetic inheritance of scent characters has remained elusive so far. In-depth knowledge of this quantitative trait is thus very much needed to breed more fragrant commercial cultivars. Furthermore, rose hybrids harbor a composite genomic structure, which complexifies quantitative trait studies. To understand rose scent inheritance, we characterized a segregating population from two diploid cultivars, Rosa x hybrida cv H190 and Rosa wichurana, which have contrasting scent profiles. Several quantitative trait loci for the major volatile compounds in this progeny were identified. One among these loci contributing to the production of 2-phenylethanol, responsible for the characteristic odor of rose, was found to be colocalized with a candidate gene belonging to the 2-phenylethanol biosynthesis pathway: the Phenylacetaldehyde SYNTHASE gene RhPAAS An in-depth allele-specific expression analysis in the progeny demonstrated that only one allele was highly expressed and was responsible for the production of 2-phenylethanol. Unexpectedly, its expression was found to start early during flower development, before the production of the volatile 2-phenylethanol, leading to the accumulation of glycosylated compounds in petals.
Honey Norisoprenoids Attract Bumble Bee, Bombus terrestris, in New Zealand Mountain Beech Forests.[Pubmed:30415534]
J Agric Food Chem. 2018 Nov 27.
Three varieties of honey of different dominant floral origin were found to attract social Hymenoptera, including the large earth bumble bee, Bombus terrestris, in a New Zealand mountain beech forest. This study was undertaken to identify volatile organic compounds that induce the attraction of bumble bees to honeybee ( Apis mellifera) honey. We analyzed the chemical composition of the volatile organic compounds produced in three distinct varieties of honey (i.e., manuka, honeydew, and clover honey). The composition of the chemical profile of the three honey varieties differed in the quality and in the ratio of compounds in the headspace. o-Methoxyacetophenone was the main compound in the headspace of all three honey varieties. Among the 40 compounds identified in the headspace in the three varieties, only seven shared compounds (i.e., benzaldehyde, benzyl alcohol, Phenylacetaldehyde, 2-phenylethanol, isophorone, 4-oxoisophorone, and o-methoxyacetophenone) were present in the headspace of the three honey varieties. The relative attractiveness of various blends of the seven common compounds found in the three honey varieties was tested for the attraction to bumble bees in a mountain beech forest. A binary blend of isophorone and 4-oxoisophorone at a ratio of 90:10 was the most attractive blend for both bumble bee workers and queens. A small number of honey bee workers were also attracted to the former binary blend. Our study represents the first identification of a honey-derived attractant for bumble bees and honey bees. The potential application of our finding for monitoring of bumble bees or to enhance crop pollination and help to tackle the current concern of a global pollination crisis is discussed.
Use of Indigenous Hanseniaspora vineae and Metschnikowia pulcherrima Co-fermentation With Saccharomyces cerevisiae to Improve the Aroma Diversity of Vidal Blanc Icewine.[Pubmed:30405538]
Front Microbiol. 2018 Oct 22;9:2303.
Using novel non-Saccharomyces strains is regarded as an effective way to improve the aroma diversity of wines to meet the expectations of consumers. The non-Saccharomyces Hanseniaspora vineae and Metschnikowia pulcherrima have good aromatic properties useful for the production of table wine. However, no detailed information is available on their performances in icewine fermentation. In this study, simultaneous and sequential fermentation trials of indigenous M. pulcherrima CVE-MP20 or H. vineae CVE-HV11 with S. cerevisiae (SC45) were performed in 50-L fermenters of Vidal icewine, respectively. The results showed that SC45 cofermented with different non-Saccharomyces strains could generate a distinct aroma quality of icewine compared with four S. cerevisiae strain monocultures as evidenced by principal component analysis (PCA). Mixed fermentation of MP20/SC45 produced higher contents of acetate esters and beta-damascenone with lower C6 alcohols relative to SC45 monoculture. Interestingly, HV11/SC45 generated the highest amounts of C6 alcohols [(Z)-3-hexen-1-ol and (E)-3-hexen-1-ol], higher alcohols (isobutanol, isopentanol, and 2-phenylethanol), acetate esters (2-phenethyl acetate and isoamyl acetate), cis-rose oxide, beta-damascenone, and Phenylacetaldehyde. Compared with simultaneous inoculation, sequential inoculation could achieve higher aroma diversity and produce higher intensity of fruity, flowery, and sweet attributes of icewine as assessed by calculating the odor activity values (OAVs). Our results verified the desired enological characteristics of H. vineae strain in icewine fermentation and also demonstrated that using indigenous non-Saccharomyces and Saccharomyces strains is a feasible way to improve aroma diversity of icewine products, which could provide an alternative way to meet the requirement of wine consumers for diversified aromatic quality.
Attraction of phlebotomine sandflies to volatiles from skin odors of individuals residing in an endemic area of tegumentary leishmaniasis.[Pubmed:30248113]
PLoS One. 2018 Sep 24;13(9):e0203989.
BACKGROUND: Many studies have investigated what could attract insects of medical importance and a crucial role has lately been attributed to human skin odors. Most of these researches have been concerned with mosquitoes, e.g., vectors of dengue and malaria. Little is known about volatile organic compounds (VOCs) from human skin odors and their effects on leishmania vectors. OBJECTIVE: The present study aimed to identify the VOCs from human skin that can be attractive to female anthropophilic phlebotomine sandflies. RESULTS: Forty-two VOCs were identified from skin odors of 33 male volunteers, seven of which were tested in wind tunnel assays employing field-captured phlebotomine sandflies (75.4% identified as Lutzomyia intermedia). Hexane and (E)-oct-3-en-1-ol (octenol) were used as negative and positive controls, respectively. 2-Phenylacetaldehyde (hereafter called Phenylacetaldehyde), 6-methylhept-5-en-2-one (also known as sulcatone), nonadecane and icosane were found to activate female phlebotomine sandflies, but only Phenylacetaldehyde, 6-methylhepten-5-en-2-one and icosane elicited attraction responses. CONCLUSIONS: These results suggest that Phenylacetaldehyde, 6-methylhepten-5-en-2-one and icosane may be suitable candidates for attractiveness experimentation in the field which can be an important tool to develop strategies concerning human beings protection against phlebotomine sandflies bites and consequently against leishmaniasis.


