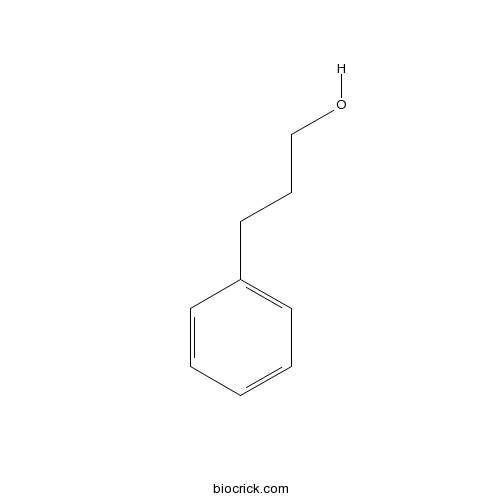
3-Phenyl-1-propanolCAS# 122-97-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122-97-4 | SDF | Download SDF |
| PubChem ID | 31234 | Appearance | Powder |
| Formula | C9H12O | M.Wt | 136 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-phenylpropan-1-ol | ||
| SMILES | C1=CC=C(C=C1)CCCO | ||
| Standard InChIKey | VAJVDSVGBWFCLW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H12O/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6,10H,4,7-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Phenyl-1-propanol Dilution Calculator

3-Phenyl-1-propanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3529 mL | 36.7647 mL | 73.5294 mL | 147.0588 mL | 183.8235 mL |
| 5 mM | 1.4706 mL | 7.3529 mL | 14.7059 mL | 29.4118 mL | 36.7647 mL |
| 10 mM | 0.7353 mL | 3.6765 mL | 7.3529 mL | 14.7059 mL | 18.3824 mL |
| 50 mM | 0.1471 mL | 0.7353 mL | 1.4706 mL | 2.9412 mL | 3.6765 mL |
| 100 mM | 0.0735 mL | 0.3676 mL | 0.7353 mL | 1.4706 mL | 1.8382 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phenylacetaldehyde
Catalog No.:BCN3819
CAS No.:122-78-1
- Cinnamyl cinnamate
Catalog No.:BCN4722
CAS No.:122-69-0
- (-)-Ampelopsin H
Catalog No.:BCC8842
CAS No.:
- Zingerone
Catalog No.:BCN1192
CAS No.:122-48-5
- Glycerine trioleate
Catalog No.:BCN2287
CAS No.:122-32-7
- Tetraethoxypropane
Catalog No.:BCN2221
CAS No.:122-31-6
- Sulfadimethoxine
Catalog No.:BCC5159
CAS No.:122-11-2
- (±)-Anatoxin A fumarate
Catalog No.:BCC6796
CAS No.:1219922-30-1
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- PKA inhibitor fragment (6-22) amide
Catalog No.:BCC1042
CAS No.:121932-06-7
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
- Sulfamonomethoxine
Catalog No.:BCC9156
CAS No.:1220-83-3
- Niazirin
Catalog No.:BCN7300
CAS No.:122001-32-5
- Cyhalofop
Catalog No.:BCC5474
CAS No.:122008-78-0
- PLP (139-151)
Catalog No.:BCC5920
CAS No.:122018-58-0
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
- Khayalenoid E
Catalog No.:BCN6111
CAS No.:1220508-29-1
- [bAla8]-Neurokinin A(4-10)
Catalog No.:BCC7137
CAS No.:122063-01-8
- 2'-O-Acetylsprengerinin C
Catalog No.:BCN6655
CAS No.:1220707-33-4
- Charantadiol A
Catalog No.:BCN3483
CAS No.:1220890-23-2
- 3,4-Dihydro-3,4-dihydroxynaphthalen-1(2H)-one
Catalog No.:BCN1602
CAS No.:1220891-22-4
- Auraptenol
Catalog No.:BCN6113
CAS No.:1221-43-8
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
Catalog No.:BCC8575
CAS No.:122111-01-7
Crystal Structure, Cytotoxicity and Interaction with DNA of Zinc (II) Complexes with o-Vanillin Schiff Base Ligands.[Pubmed:26114437]
PLoS One. 2015 Jun 26;10(6):e0130922.
Two new zinc complexes, Zn(HL1)2 (1) and [Zn2(H2L2)(OAc)2]2 (2) [H2L1 = Schiff base derived from o-vanillin and (R)-(+)-2-amino-3-Phenyl-1-propanol, H3L2 = Schiff base derived from o-vanillin and 2-amino-2-ethyl-1,3-propanediol], have been synthesized and characterized by single crystal X-ray diffraction, elemental analyses, TG analyses, solid fluorescence, IR, UV-Vis and circular dichroism spectra. The structural analysis shows that complex 1 has a right-handed double helical chain along the crystallographic b axis. A homochiral 3D supramolecular architecture has been further constructed by intermolecular C-H... pi, O-H...O and C-H...O interactions. Complex 2 includes two crystallographically independent binuclear zinc molecules. The two binuclear zinc molecules are isostructural. The 2-D sheet supramolecular structure was formed by intermolecular hydrogen bonding interaction. The fluorescence of ligands and complexes in DMF at room temperature are studied. The interactions of two complexes with calf thymus DNA (CT-DNA) are investigated using UV-Vis, CD and fluorescence spectroscopy. The results show that complex 1 exhibits higher interaction with CT-DNA than complex 2. In addition, in vitro cytotoxicity of the complexes towards four kinds of cancerous cell lines (A549, HeLa, HL-60 and K562) were assayed by the MTT method. Investigations on the structures indicated that the chirality and nuclearity of zinc complexes play an important role on cytotoxic activity.
The Amino Acid Specificity for Activation of Phenylalanine Hydroxylase Matches the Specificity for Stabilization of Regulatory Domain Dimers.[Pubmed:26252467]
Biochemistry. 2015 Aug 25;54(33):5167-74.
Liver phenylalanine hydroxylase is allosterically activated by phenylalanine. The structural changes that accompany activation have not been identified, but recent studies of the effects of phenylalanine on the isolated regulatory domain of the enzyme support a model in which phenylalanine binding promotes regulatory domain dimerization. Such a model predicts that compounds that stabilize the regulatory domain dimer will also activate the enzyme. Nuclear magnetic resonance spectroscopy and analytical ultracentrifugation were used to determine the ability of different amino acids and phenylalanine analogues to stabilize the regulatory domain dimer. The abilities of these compounds to activate the enzyme were analyzed by measuring their effects on the fluorescence change that accompanies activation and on the activity directly. At concentrations of 10-50 mM, d-phenylalanine, l-methionine, l-norleucine, and (S)-2-amino-3-Phenyl-1-propanol were able to activate the enzyme to the same extent as 1 mM l-phenylalanine. Lower levels of activation were seen with l-4-aminophenylalanine, l-leucine, l-isoleucine, and 3-phenylpropionate. The ability of these compounds to stabilize the regulatory domain dimer agreed with their ability to activate the enzyme. These results support a model in which allosteric activation of phenylalanine hydroxylase is linked to dimerization of regulatory domains.
An expedient synthesis of maraviroc (UK-427,857) via C-H functionalization.[Pubmed:26120216]
Tetrahedron Lett. 2015 Jun 3;56(23):3620-3623.
A new, concise synthesis of the CCR-5 receptor antagonist maraviroc (UK-427,857) from 3-Phenyl-1-propanol has been completed in four steps featuring a site-selective C-H functionalization.
Bacterial Catabolism of beta-Hydroxypropiovanillone and beta-Hydroxypropiosyringone Produced in the Reductive Cleavage of Arylglycerol-beta-Aryl Ether in Lignin.[Pubmed:29374031]
Appl Environ Microbiol. 2018 Mar 19;84(7). pii: AEM.02670-17.
Sphingobium sp. strain SYK-6 converts four stereoisomers of arylglycerol-beta-guaiacyl ether into achiral beta-hydroxypropiovanillone (HPV) via three stereospecific reaction steps. Here, we determined the HPV catabolic pathway and characterized the HPV catabolic genes involved in the first two steps of the pathway. In SYK-6 cells, HPV was oxidized to vanilloyl acetic acid (VAA) via vanilloyl acetaldehyde (VAL). The resulting VAA was further converted into vanillate through the activation of VAA by coenzyme A. A syringyl-type HPV analog, beta-hydroxypropiosyringone (HPS), was also catabolized via the same pathway. SLG_12830 (hpvZ), which belongs to the glucose-methanol-choline oxidoreductase family, was isolated as the HPV-converting enzyme gene. An hpvZ mutant completely lost the ability to convert HPV and HPS, indicating that hpvZ is essential for the conversion of both the substrates. HpvZ produced in Escherichia coli oxidized both HPV and HPS and other 3-Phenyl-1-propanol derivatives. HpvZ localized to both the cytoplasm and membrane of SYK-6 and used ubiquinone derivatives as electron acceptors. Thirteen gene products of the 23 aldehyde dehydrogenase (ALDH) genes in SYK-6 were able to oxidize VAL into VAA. Mutant analyses suggested that multiple ALDH genes, including SLG_20400, contribute to the conversion of VAL. We examined whether the genes encoding feruloyl-CoA synthetase (ferA) and feruloyl-CoA hydratase/lyase (ferB and ferB2) are involved in the conversion of VAA. Only FerA exhibited activity toward VAA; however, disruption of ferA did not affect VAA conversion. These results indicate that another enzyme system is involved in VAA conversion.IMPORTANCE Cleavage of the beta-aryl ether linkage is the most essential process in lignin biodegradation. Although the bacterial beta-aryl ether cleavage pathway and catabolic genes have been well documented, there have been no reports regarding the catabolism of HPV or HPS, the products of cleavage of beta-aryl ether compounds. HPV and HPS have also been found to be obtained from lignin by chemoselective catalytic oxidation by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone/tert-butyl nitrite/O2, followed by cleavage of the beta-aryl ether with zinc. Therefore, value-added chemicals are expected to be produced from these compounds. In this study, we determined the SYK-6 catabolic pathways for HPV and HPS and identified the catabolic genes involved in the first two steps of the pathways. Since SYK-6 catabolizes HPV through 2-pyrone-4,6-dicarboxylate, which is a building block for functional polymers, characterization of HPV catabolism is important not only for understanding the bacterial lignin catabolic system but also for lignin utilization.
Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae).[Pubmed:27742359]
Pestic Biochem Physiol. 2016 Oct;133:35-43.
Fumigant and contact toxicities of 22 plant essential oils (EOs) from 14 families and their constituents against the adult spotted wing drosophila (SWD), Drosophila suzukii were examined. Analyses by GC, GC-MS, and NMR led to the identification of 2, 16, 13, 4, 6, 9, and 10 compounds from Gaultheria fragrantissima, Croton anistatum, Illicium verum, Liquidamabar orientalis, Cinnamomum cassia, Rosa damasena, and Santalum album, respectively. In fumigant toxicity test, G. fragrantissima, C. anistatum, and I. verum exhibited 100, 93.8, and 95.8, and 100, 70.0, and 80.0% mortalities against the adult male and female SWD at 4.41mg/L air, respectively. LC50 values (mg/L air) of G. fragrantissima, C. anistatum, and I. verum were 3.46, 3.67, and 3.16 against male, and 3.48, 4.31, and 4.01 against female SWD. LC50 values (mg/L air) of methyl salicylate and trans-anethole were 2.17 and 1.75 against male and 2.65 and 3.00 against female SWD, respectively. In contact toxicity tests, L. orientalis, C. cassia, R. damasena, and S. album showed insecticidal activity with LD50 values (mug/fly) of 2.64, 1.84, 3.40 and 2.18 against male SWD and of 3.74, 2.24, 8.91 and 5.61 against female SWD, respectively. 2-Phehy-1-ethanol, 3-Phenyl-1-propanol, trans-cinnamaldehyde, trans-cinnamyl alcohol, and alpha-santalol also exhibited insecticidal activity with LD50 values of 9.79, 5.52, 2.39, 3.02 and 2.37 against male SWD and of 11.77, 7.04, 2.94, 3.32, and 3.99 against female SWD, respectively. trans-Cinnamaldehyde exhibited the highest AChE inhibition but its inhibition is likely due to a non-specific chemical inhibition. Our results indicate that wooden EOs and their components can be used as fumigants or spray-type control agents against SWD.
One-step synthesis of hybrid nanocrystals with rational tuning of the morphology.[Pubmed:25310606]
Nano Lett. 2014 Nov 12;14(11):6666-71.
Metal-sulfide hybrid nanocrystals (HNCs) have been of great interest for their distinguished interfacial effect, which gives rise to unique catalytic properties. However, most of the reported metal-sulfide HNCs were synthesized via two-step approaches and few were fabricated based on the one-step strategies. Herein, we report a facile one-pot synthesis of CuPt-Cu2S, Pt-Cu2S HNCs, and CuPt nanocubes by simply changing the Pt precursor types. 1-Hexadecanethiol (HDT) was employed in this system to mediate the reduction of metal precursors and also as capping agent and sulfur source. Moreover, CuPd-Cu2S and Au-Cu2S HNCs were successfully prepared by using this one-step method. The catalytic properties of the obtained three nanocrystals were investigated in hydrogenation of cinnamaldehyde. Results show that CuPt-Cu2S HNCs exhibited the highest conversion rate and the highest selectivity toward hydrocinnamaldehyde while 3-Phenyl-1-propanol was the only product over Pt-Cu2S HNCs.


