Phenyl benzoateCAS# 93-99-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93-99-2 | SDF | Download SDF |
| PubChem ID | 7169 | Appearance | Powder |
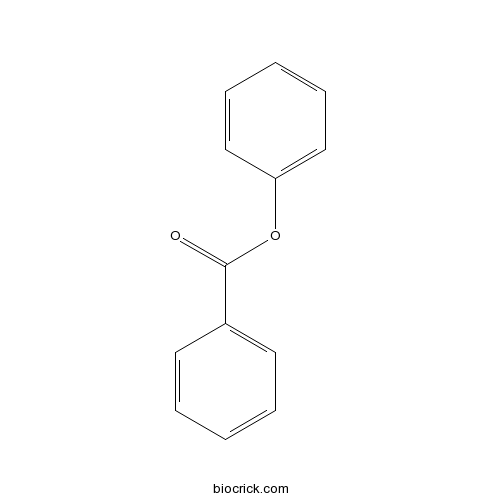
| Formula | C13H10O2 | M.Wt | 198.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | phenyl benzoate | ||
| SMILES | C1=CC=C(C=C1)C(=O)OC2=CC=CC=C2 | ||
| Standard InChIKey | FCJSHPDYVMKCHI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10O2/c14-13(11-7-3-1-4-8-11)15-12-9-5-2-6-10-12/h1-10H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phenyl benzoate Dilution Calculator

Phenyl benzoate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0449 mL | 25.2245 mL | 50.449 mL | 100.898 mL | 126.1225 mL |
| 5 mM | 1.009 mL | 5.0449 mL | 10.0898 mL | 20.1796 mL | 25.2245 mL |
| 10 mM | 0.5045 mL | 2.5224 mL | 5.0449 mL | 10.0898 mL | 12.6122 mL |
| 50 mM | 0.1009 mL | 0.5045 mL | 1.009 mL | 2.018 mL | 2.5224 mL |
| 100 mM | 0.0504 mL | 0.2522 mL | 0.5045 mL | 1.009 mL | 1.2612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzanilide
Catalog No.:BCC8844
CAS No.:93-98-1
- 1-Phenylbutane-1,3-dione
Catalog No.:BCN3807
CAS No.:93-91-4
- Skimmin
Catalog No.:BCN4479
CAS No.:93-39-0
- Umbelliferone
Catalog No.:BCN4477
CAS No.:93-35-6
- Acetylisoeugenol
Catalog No.:BCN7075
CAS No.:93-29-8
- N-(2-Methoxyphenyl)acetamide
Catalog No.:BCC9054
CAS No.:93-26-5
- Methyl isoeugenol
Catalog No.:BCN8462
CAS No.:93-16-3
- Methyleugenol
Catalog No.:BCN4074
CAS No.:93-15-2
- Guaifenesin
Catalog No.:BCN2977
CAS No.:93-14-1
- 2-Acetonaphthone
Catalog No.:BCC8510
CAS No.:93-08-3
- 3,4-Dimethoxybenzoic acid
Catalog No.:BCN4475
CAS No.:93-07-2
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
- Isosalvianolic Acid B
Catalog No.:BCC8330
CAS No.:930573-88-9
- R 59-022
Catalog No.:BCC7279
CAS No.:93076-89-2
- 8-O-Demethyl-7-O-methyl-3,9-dihydropunctatin
Catalog No.:BCN1307
CAS No.:93078-83-2
- Kaempferol 3-sophoroside-7-rhamnoside
Catalog No.:BCN1306
CAS No.:93098-79-4
- Enrofloxacin
Catalog No.:BCC4657
CAS No.:93106-60-6
- Ciprofloxacin hydrochloride
Catalog No.:BCC8915
CAS No.:93107-08-5
- (S,E)-Deca-2,9-diene-4,6-diyne-1,8-diol
Catalog No.:BCN1305
CAS No.:931114-98-6
- (R,E)-Deca-2-ene-4,6-diyne-1,8-diol
Catalog No.:BCN4476
CAS No.:931116-24-4
- Tacalcitol monohydrate
Catalog No.:BCC1976
CAS No.:93129-94-3
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
- PPQ-102
Catalog No.:BCC5248
CAS No.:931706-15-9
- 2-Hydroxybenzylamine
Catalog No.:BCN1803
CAS No.:932-30-9
Identification of an Alarm Pheromone-Binding Chemosensory Protein From the Invasive Sycamore Lace Bug Corythucha ciliata (Say).[Pubmed:29681864]
Front Physiol. 2018 Apr 6;9:354.
The spread of the exotic insect pest sycamore lace bug Corythucha ciliata (Say) is increasing worldwide. The identification of behaviorally active compounds is crucial for reducing the current distribution of this pest. In this study, we identified and documented the expression profiles of genes encoding chemosensory proteins (CSPs) in the sycamore lace bug to identify CSPs that bind to the alarm pheromone geraniol. One CSP (CcilCSP2) that was highly expressed in nymph antennae was found to bind geraniol with high affinity. This finding was confirmed by fluorescence competitive binding assays. We further discovered one candidate chemical, Phenyl benzoate, that bound to CcilCSP2 with even higher affinity than geraniol. Behavioral assays revealed that Phenyl benzoate, similar to geraniol, significantly repelled sycamore lace bug nymphs but had no activity toward adults. This study has revealed a novel repellent compound involved in behavioral regulation. And, our findings will be beneficial for understanding the olfactory recognition mechanism of sycamore lace bug and developing a push-pull system to manage this pest in the future.
A cytotoxicity, optical spectroscopy and computational binding analysis of 4-[3-acetyl-5-(acetylamino)-2-methyl-2,3-dihydro-1,3,4-thiadiazole-2-yl]phenyl benzoate in calf thymus DNA.[Pubmed:29578306]
Luminescence. 2018 Jun;33(4):731-741.
In this study the interaction mechanism between newly synthesized 4-(3-acetyl-5-(acetylamino)-2-methyl-2, 3-dihydro-1,3,4-thiadiazole-2-yl) Phenyl benzoate (thiadiazole derivative) anticancer active drug with calf thymus DNA was investigated by using various optical spectroscopy techniques along with computational technique. The absorption spectrum shows a clear shift in the lower wavelength region, which may be due to strong hypochromic effect in the ctDNA and the drug. The results of steady state fluorescence spectroscopy show that there is static quenching occurring while increasing the thiadiazole drug concentration in the ethidium bromide-ctDNA system. Also the binding constant (K), thermo dynamical parameters of enthalpy change (DeltaH degrees ), entropy change (DeltaS degrees ) Gibbs free energy change (DeltaG degrees ) were calculated at different temperature (293 K, 298 K) and the results are in good agreement with theoretically calculated MMGBSA binding analysis. Time resolved emission spectroscopy analysis clearly explains the thiadiazole derivative competitive intercalation in the ethidium bromide-ctDNA system. Further, molecular docking studies was carried out to understand the hydrogen bonding and hydrophobic interaction between ctDNA and thiadiazole derivative molecule. In addition the docking and molecular dynamics charge distribution analysis was done to understand the internal stability of thiadiazole derivative drug binding sites of ctDNA. The global reactivity of thiadiazole derivative such as electronegativity, electrophilicity and chemical hardness has been calculated.


