Methyl isoeugenolCAS# 93-16-3 |
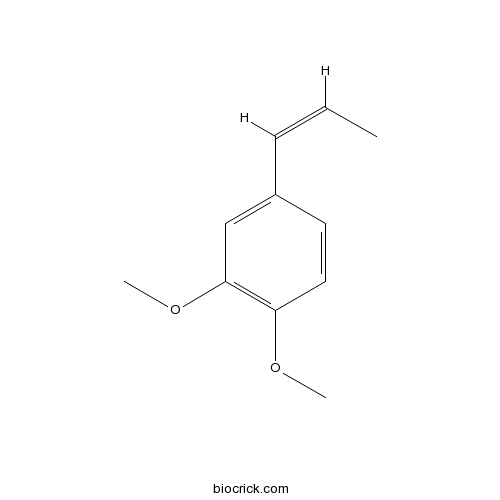
- trans-Methylisoeugenol
Catalog No.:BCN6558
CAS No.:6379-72-2
- cis-Methylisoeugenol
Catalog No.:BCN0620
CAS No.:6380-24-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93-16-3 | SDF | Download SDF |
| PubChem ID | 1549045 | Appearance | Colorless liquid |
| Formula | C11H14O2 | M.Wt | 178.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Isomethyleugenol;6380-24-1 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2-dimethoxy-4-[(Z)-prop-1-enyl]benzene | ||
| SMILES | CC=CC1=CC(=C(C=C1)OC)OC | ||
| Standard InChIKey | NNWHUJCUHAELCL-PLNGDYQASA-N | ||
| Standard InChI | InChI=1S/C11H14O2/c1-4-5-9-6-7-10(12-2)11(8-9)13-3/h4-8H,1-3H3/b5-4- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl isoeugenol Dilution Calculator

Methyl isoeugenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6107 mL | 28.0536 mL | 56.1073 mL | 112.2146 mL | 140.2682 mL |
| 5 mM | 1.1221 mL | 5.6107 mL | 11.2215 mL | 22.4429 mL | 28.0536 mL |
| 10 mM | 0.5611 mL | 2.8054 mL | 5.6107 mL | 11.2215 mL | 14.0268 mL |
| 50 mM | 0.1122 mL | 0.5611 mL | 1.1221 mL | 2.2443 mL | 2.8054 mL |
| 100 mM | 0.0561 mL | 0.2805 mL | 0.5611 mL | 1.1221 mL | 1.4027 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyleugenol
Catalog No.:BCN4074
CAS No.:93-15-2
- Guaifenesin
Catalog No.:BCN2977
CAS No.:93-14-1
- 2-Acetonaphthone
Catalog No.:BCC8510
CAS No.:93-08-3
- 3,4-Dimethoxybenzoic acid
Catalog No.:BCN4475
CAS No.:93-07-2
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
- Cucumegastigmane I
Catalog No.:BCN4474
CAS No.:929881-46-9
- Sessilifoline A
Catalog No.:BCN4473
CAS No.:929637-35-4
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Fmoc-Tyr-OH
Catalog No.:BCC3562
CAS No.:92954-90-0
- LUF 6283
Catalog No.:BCC6318
CAS No.:92933-48-7
- N-(2-Methoxyphenyl)acetamide
Catalog No.:BCC9054
CAS No.:93-26-5
- Acetylisoeugenol
Catalog No.:BCN7075
CAS No.:93-29-8
- Umbelliferone
Catalog No.:BCN4477
CAS No.:93-35-6
- Skimmin
Catalog No.:BCN4479
CAS No.:93-39-0
- 1-Phenylbutane-1,3-dione
Catalog No.:BCN3807
CAS No.:93-91-4
- Benzanilide
Catalog No.:BCC8844
CAS No.:93-98-1
- Phenyl benzoate
Catalog No.:BCN8522
CAS No.:93-99-2
- Isosalvianolic Acid B
Catalog No.:BCC8330
CAS No.:930573-88-9
- R 59-022
Catalog No.:BCC7279
CAS No.:93076-89-2
- 8-O-Demethyl-7-O-methyl-3,9-dihydropunctatin
Catalog No.:BCN1307
CAS No.:93078-83-2
- Kaempferol 3-sophoroside-7-rhamnoside
Catalog No.:BCN1306
CAS No.:93098-79-4
- Enrofloxacin
Catalog No.:BCC4657
CAS No.:93106-60-6
[Study on GC-MS fingerprint analysis in rhizome of volatile oil of Acorus tatarinowii].[Pubmed:15506289]
Zhongguo Zhong Yao Za Zhi. 2004 Aug;29(8):764-8.
OBJECTIVE: To establish the method of fingerprint analysis on volatile oil in rhizome of Acorus tatarinowii by GC-MS, and to study the main characteristic components. METHOD: The main components of 10 samples were determined by GC-MS. RESULT: The injector temperature was 250 degrees C. The interface temperature was 230 degrees C. The column flow was 1.3 mL x min(-1). The column pressure was 80 kPa. The detector volt was 1.4 kV. The temperature rate was 3 degrees C x min(-1). And the main characteristic components were composed of the methyleugenol (2.13%), cis-methylisoeugenol (4.48%), trans-methylisoeugenol (0.82%), gamma-asarone (4.51%), beta-asarone (66.15%), alpha-asarone (6.35%). And the RSD of precision and reproducibility and stability was almost in the range of 5%. CONCLUSION: The method is reliable, accurate and can be used for fingerprint analysis of volatile oil of Acorus tatarinowii.
Clausena anisata and Dysphania ambrosioides essential oils: from ethno-medicine to modern uses as effective insecticides.[Pubmed:28965298]
Environ Sci Pollut Res Int. 2018 Apr;25(11):10493-10503.
Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) and Clausena anisata (Willd.) Hook. f. ex Benth. (Rutaceae) are two aromatic species traditionally used in Cameroon to repel and kill insects. The present work was carried out to substantiate this traditional use and to evaluate the possible incorporation in commercial botanical insecticides of their essential oils (EOs). The EOs were distilled from leaves of C. anisata and aerial parts of D. ambrosioides and analyzed by gas chromatography-mass spectrometry (GC-MS). The insecticidal activity of both EOs was investigated against the filariasis vector, Culex quinquefasciatus, and the housefly, Musca domestica. As possible mode of action, the inhibition of acetylcholinesterase (AChE) by the two EOs was investigated as well. The D. ambrosioides EO was characterized by the monoterpene peroxide ascaridole (61.4%) and the aromatic p-cymene (29.0%), whereas the C. anisata EO was dominated by the phenylpropanoids (E)-anethole (64.6%) and (E)-Methyl isoeugenol (16.1%). The C. anisata EO proved to be very toxic to third instar larvae of C. quinquefasciatus showing LC50 of 29.3 mul/l, whereas D. ambrosioides EO was more toxic to adults of M. domestica showing a LD50 of 51.7 mug/adult. The mixture of both EOs showed a significant synergistic effect against mosquito larvae with LC50 estimated as 19.3 mul/l, whereas this phenomenon was not observed upon application to M. domestica adults (LD50 = 75.9 mug/adult). Of the two EOs, the D. ambrosioides one provided a good inhibition of AChE (IC50 = 77 mug/ml), whereas C. anisata oil was not effective. These findings provide new evidences supporting the ethno-botanical use of these two Cameroonian plants, and their possible application even in synergistic binary blends, to develop new eco-friendly, safe and effective herbal insecticides.
Seasonal Variation in Essential Oil Compositions and Antioxidant Properties of Acorus calamus L. Accessions.[Pubmed:29117116]
Medicines (Basel). 2017 Nov 8;4(4). pii: medicines4040081.
Background:Acorus calamus (Sweet flag) is a known herbal drug commonly used in traditional medicine. Our aim was to perform seasonal and altitudinal phytochemical screening to assess the antioxidant activity of the essential oils in the rhizome and leaves of A. calamus from three different altitudes. Methods: Phytochemical screening was performed using GC/MS analysis and in vitro antioxidant assay was done by different methods. Results: The essential oils mainly contained alpha-asarone, beta-asarone (35.3-90.6%), and Z-isoelemicin (1.7-7.3%) as the major constituents, besides linalool, Z-Methyl isoeugenol, shyobunone, kessane, etc. All the oils exhibited vast molecular diversity in terms of quantitative ingredients. All essential oils were studied for their antioxidant activity by different methods, including their effect on the DPPH radical-scavenging activity, reducing power, and chelating properties of Fe(2+). The oils isolated in all the different seasons exhibited antioxidant activity as a function of concentration, with IC50 values ranging from 475.48 +/- 0.08 to 11.72 +/- 0.03 compared to standards. Conclusion : From the results obtained it can be inferred that the herb may be a good source of bioactive compounds and can work as an antioxidant to prevent oxidative deterioration in food. The data provide a basis for its in-situ investigation for judicious exploitation.
Toxicity of Plant Secondary Metabolites Modulating Detoxification Genes Expression for Natural Red Palm Weevil Pesticide Development.[Pubmed:28117698]
Molecules. 2017 Jan 20;22(1). pii: molecules22010169.
This study aimed to explore the larvicidal and growth-inhibiting activities, and underlying detoxification mechanism of red palm weevil against phenylpropanoids, an important class of plant secondary metabolites. Toxicity of alpha-asarone, eugenol, isoeugenol, methyl eugenol, Methyl isoeugenol, coumarin, coumarin 6, coniferyl aldehyde, diniconazole, ethyl cinnamate, and rosmarinic acid was evaluated by incorporation into the artificial diet. All of the phenylpropanoids exhibited dose- and time-dependent insecticidal activity. Among all the tested phenylpropanoids, coumarin exhibited the highest toxicity by revealing the least LD50 value (0.672 g/L). In addition, the most toxic compound (coumarin) observed in the current study, deteriorated the growth resulting tremendous reduction (78.39%) in efficacy of conversion of digested food (ECD), and (ECI) efficacy of conversion of ingested food (70.04%) of tenth-instar red palm weevil larvae. The energy-deficient red palm weevil larvae through their intrinsic abilities showed enhanced response to their digestibility resulting 27.78% increase in approximate digestibility (AD) compared to control larvae. The detoxification response of Rhynchophorus ferrugineus larvae determined by the quantitative expression of cytochrome P450, esterases, and glutathione S-transferase revealed enhanced expression among moderately toxic and ineffective compounds. These genes especially cytochrome P450 and GST detoxify the target compounds by enhancing their solubility that leads rapid excretion and degradation resulting low toxicity towards red palm weevil larvae. On the other hand, the most toxic (coumarin) silenced the genes involved in the red palm weevil detoxification mechanism. Based on the toxicity, growth retarding, and masking detoxification activities, coumarin could be a useful future natural red palm weevil-controlling agent.
Structure-Activity Relationships for DNA Damage by Alkenylbenzenes in Turkey Egg Fetal Liver.[Pubmed:26719370]
Toxicol Sci. 2016 Apr;150(2):301-11.
Certain alkenylbenzenes (AB), flavoring chemicals naturally occurring in spices and herbs, are established to be cytotoxic and hepatocarcinogenic in rodents. The purpose of the present study was to determine the DNA damaging potential of key representatives of this class using the Turkey Egg Genotoxicity Assay. Medium white turkey eggs with 22- to 24-day-old fetuses received three injections of nine AB with different carcinogenic potentials: safrole (1, 2 mg/egg), methyl eugenol (2, 4 mg/egg), estragole (20, 40 mg/egg), myristicin (25, 50 mg/egg), elemicin (20, 50 mg/egg), anethole (5, 10 mg/egg), Methyl isoeugenol (40, 80 mg/egg), eugenol (1, 2.5 mg/egg), and isoeugenol (1, 4 mg/egg). Three hours after the last injection, fetal livers were harvested for measurement of DNA strand breaks, using the comet assay and DNA adducts formation, using the nucleotide(3) (2)P-postlabeling assay. Estragole, myristicin, and elemicin induced DNA stand breaks. These compounds as well as safrole, methyl eugenol and anethole, at the highest doses tested, induced DNA adduct formation. Methyl isoeugenol, eugenol, and isoeugenol did not induce genotoxicity. The genotoxic AB all had the structural features of either a double bond in the alkenyl side chain at the terminal 2',3'-position, favorable to formation of proximate carcinogenic 1'-hydroxymetabolite or terminal epoxide, or the absence of a free phenolic hydroxyl group crucial for formation of a nontoxic glucuronide conjugate. In contrast, Methyl isoeugenol, eugenol and isoeugenol, which were nongenotoxic, possessed chemical features, unfavorable to activation.
Antioxidant and antibacterial activities of Pelargonium asperum and Ormenis mixta essential oils and their synergistic antibacterial effect.[Pubmed:28735475]
Environ Sci Pollut Res Int. 2018 Oct;25(30):29860-29867.
In this work, the chemical composition, the antioxidant, and the antibacterial activities of two Moroccan essential oils less studied, extracted from Pelargonium asperum and Ormenis mixta, were investigated. According to the gas chromatography coupled to mass spectrometry analysis, citronellol (25.07%), citronellyl ester (10.52%), geraniol (10.46%), and buthyl anthranilate (5.93%) were found to be the major components of P. asperum, while O. mixta was mainly composed of D-germacrene (11.46%), 1,8-cineole (10.28%), and cis-Methyl isoeugenol (9.04%). Moreover, O. mixta essential oil exhibited an important antioxidant activity being significantly higher than that exhibited by P. asperum oil (P < 0.001). As regards the antimicrobial activity of both essential oils, the zones of growth inhibition and the minimum inhibitory concentration values showed that P. asperum essential oil was more active than that of O. mixta. Thereafter, the impact of the binary combination of essential oils on their antimicrobial effect was investigated against Staphylococcus aureus using the fractional inhibitory concentration index calculation. The results showed a promising synergistic antibacterial interaction between essential oils studied.
Developmental toxicity evaluation of Pimenta pseudocaryophyllus (Gomes) Landrum, (E)-methyl isoeugenol chemotype, in Wistar rats.[Pubmed:28762666]
Birth Defects Res. 2017 Oct 2;109(16):1292-1300.
BACKGROUND: Pimenta pseudocaryophyllus (Gomes) Landrum (Myrtaceae) has been traditionally used in Brazilian folk medicine. Studies have established the botanical characterization, phytochemistry profile, and pharmacological potential of this species, including antibiotic, anxiolytic, antidepressant, antioxidant, antinociceptive, and anti-inflammatory properties. Despite its widespread use, no previous study has been conducted regarding its toxicological profile, especially during pregnancy. Thus, this study investigated the developmental toxicity of the dry leaf extract of the P. pseudocaryophyllus, (E)-Methyl isoeugenol chemotype, in rats. METHODS: First, the dry leaf extract was prepared by a spray-drying technique. Then, pregnant Wistar rats were orally treated with dry extract at doses of 0, 2000, 2500, or 3000 mg/kg from gestational day 6 through 15 (organogenesis period). On gestational day 21, the rats underwent cesarean sections and the reproductive outcomes and biochemistry parameters related to hepatic and renal markers were evaluated. Additionally, the fetuses were examined for external and skeletal variations and malformations. RESULTS: The spray-drying technique preserved the phytocomplex components and showed a satisfactory yield. No relevant differences were seen in the food consumption, reproductive performances, and hepatic and renal biochemical parameters between groups. However, there was a decrease in body weight gain of the dams during the organogenesis period and an increase of minor skeletal variations in the offspring (increased fetal incidences only of delayed ossification of the metacarpals, metatarsals, phalanges, sternebra, and rudimentary ribs) treated with the dry extract. CONCLUSION: The extract of P. pseudocaryophyllus, (E)-Methyl isoeugenol chemotype, showed low maternal toxicity and induced minor skeletal variations in the offspring. Birth Defects Research 109:1292-1300, 2017. (c) 2017 Wiley Periodicals, Inc.


