PellitorineCAS# 18836-52-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18836-52-7 | SDF | Download SDF |
| PubChem ID | 5318516 | Appearance | Powder |
| Formula | C14H25NO | M.Wt | 223.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
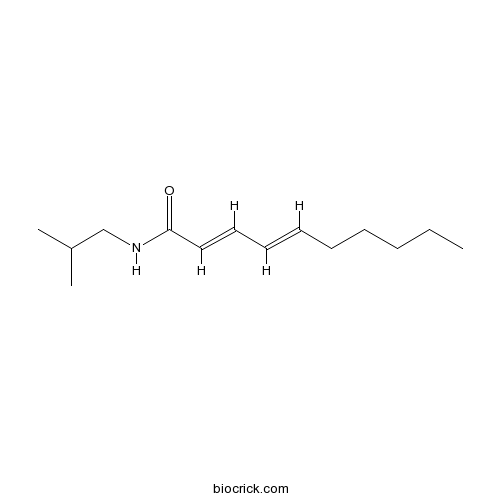
| Chemical Name | (2E,4E)-N-(2-methylpropyl)deca-2,4-dienamide | ||
| SMILES | CCCCCC=CC=CC(=O)NCC(C)C | ||
| Standard InChIKey | MAGQQZHFHJDIRE-BNFZFUHLSA-N | ||
| Standard InChI | InChI=1S/C14H25NO/c1-4-5-6-7-8-9-10-11-14(16)15-12-13(2)3/h8-11,13H,4-7,12H2,1-3H3,(H,15,16)/b9-8+,11-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pellitorine shows strong cytotoxic activities against HL60 and MCT-7 cell lines. 2. Pellitorine can suppress expression of inducible NO synthase and cyclooxygenase-2. 3. Pellitorine shows antiprotozoal activity against Plasmodium falciparum (IC50 = 3.3 ug/mL). 4. Pellitorine is a potential larvicide with a specific target site and a lead molecule for the control of mosquito populations. 5. Pellitorine shows potent antiplatelet aggregation activity. |
| Targets | NOS | COX | Nrf2 | NADPH-oxidase | HO-1 | ATPase | Antifection |

Pellitorine Dilution Calculator

Pellitorine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4763 mL | 22.3814 mL | 44.7628 mL | 89.5255 mL | 111.9069 mL |
| 5 mM | 0.8953 mL | 4.4763 mL | 8.9526 mL | 17.9051 mL | 22.3814 mL |
| 10 mM | 0.4476 mL | 2.2381 mL | 4.4763 mL | 8.9526 mL | 11.1907 mL |
| 50 mM | 0.0895 mL | 0.4476 mL | 0.8953 mL | 1.7905 mL | 2.2381 mL |
| 100 mM | 0.0448 mL | 0.2238 mL | 0.4476 mL | 0.8953 mL | 1.1191 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
Novel histopathological and molecular effects of natural compound pellitorine on larval midgut epithelium and anal gills of Aedes aegypti.[Pubmed:24260359]
PLoS One. 2013 Nov 18;8(11):e80226.
The yellow fever mosquito, Aedes aegypti, is a vector for transmitting dengue fever and yellow fever. In this study, we assessed the histopathological and molecular effects of Pellitorine, an isobutylamide alkaloid, on the third instar of Ae. aegypti larvae. At 5 mg/l concentration of Pellitorine, the whole body of the treated larvae became dark in color, particularly damaged thorax and abdominal regions. Pellitorine was targeted mainly on midgut epithelium and anal gills, indicating variably dramatic degenerative responses of the midgut through a sequential epithelial disorganization. The anterior and posterior midgut was entirely necrosed, bearing only gut lumen residues inside the peritrophic membranes. Pellitorine caused comprehensive damage of anal gill cells and branches of tracheole and debris was found in hemolymph of the anal gills. RT-PCR analysis indicates that the compound inhibited gene expression encoding V-type H(+)-ATPase and aquaporine 4 after treatment with 2.21 mg/l Pellitorine. These results verify that Pellitorine merits further study as a potential larvicide with a specific target site and a lead molecule for the control of mosquito populations.
Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO-1 Pathway.[Pubmed:28185326]
Phytother Res. 2017 Apr;31(4):663-670.
In the present study, ten alkaloids, namely chabamide (1), Pellitorine (2), retrofractamide A (3), pyrroperine (4), isopiperolein B (5), piperamide C9:1 (8E) (6), 6,7-dehydrobrachyamide B (7), 4,5-dihydropiperine (8), dehydropipernonaline (9), and piperine (10), were isolated from the fruits of Piper nigrum. Among these, chabamide (1), Pellitorine (2), retrofractamide A (3), isopiperolein B (5), and 6,7-dehydrobrachyamide B (7) exhibited significant inhibitory activity on lipopolysaccharide-induced nitric oxide (NO) production in RAW264.7 cells, with IC50 values of 6.8, 14.5, 30.2, 23.7, and 38.5 muM, respectively. Furthermore, compound 1 inhibited lipopolysaccharide-induced NO production in bone marrow-derived macrophages with IC50 value of 9.5 muM. Consistent with NO inhibition, treatment of RAW264.7 cells with chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) suppressed expression of inducible NO synthase and cyclooxygenase-2. Chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) induced heme-oxygenase-1 expression at the transcriptional level. In addition, compound 1 induced the nuclear translocation of nuclear factor-E2-related factor 2 (Nrf2) and upregulated the expression of Nrf2 target genes, NAD(P)H:quinone oxidoreductase 1 and gamma-glutamyl cysteine synthetase catalytic subunit, in a concentration-dependent manner in RAW264.7 cells. These findings suggest that chabamide (1) from P. nigrum exert antiinflammatory effects via the activation of the Nrf2/heme-oxygenase-1 pathway; hence, it might be a promising candidate for the treatment of inflammatory diseases. Copyright (c) 2017 John Wiley & Sons, Ltd.
Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum.[Pubmed:20428051]
Molecules. 2010 Apr 5;15(4):2398-404.
Pellitorine (1), which was isolated from the roots of Piper nigrum, showed strong cytotoxic activities against HL60 and MCT-7 cell lines. Microbial transformation of piperine (2) gave a new compound 5-[3,4-(methylenedioxy)phenyl]-pent-2-ene piperidine (3). Two other alkaloids were also found from Piper nigrum. They are (E)-1-[3',4'-(methylenedioxy)cinnamoyl]piperidine (4) and 2,4-tetradecadienoic acid isobutyl amide (5). These compounds were isolated using chromatographic methods and their structures were elucidated using MS, IR and NMR techniques.
Quantitative transdermal behavior of pellitorine from Anacyclus pyrethrum extract.[Pubmed:25481393]
Phytomedicine. 2014 Dec 15;21(14):1801-7.
The plant Anacyclus pyrethrum (AP) consists of several N-alkylamides with Pellitorine as main constituent. AP extracts are known to be biologically active and some products for topical administration containing AP plant extracts are already commercially available with functional cosmeceutical claims. However, no transdermal data for Pellitorine are currently available. Therefore, our general goal was to investigate the local skin pharmacokinetics of the plant N-alkylamide Pellitorine using a Franz diffusion cell set-up. Two different forms were applied on human skin: purified Pellitorine and the AP extract. Our study demonstrated that Pellitorine is able to cross the stratum corneum and the subsequent skin layers. A significantly higher permeability coefficient was observed when the AP extract (Kp=2.3 x 10(-4)cm/h) was administered, compared to purified Pellitorine (Kp=1.1 x 10(-4)cm/h). With the obtained Pellitorine concentrations in the skin layers and the receptor fluid, it is concluded that local and systemic effects can be expected after topical application. Due to these findings and as a regulatory consequence, products containing reasonable concentrations of Pellitorine are recommended to be classified as a medicinal product.
Isolation and identification of antiplatelet aggregatory principles from the leaves of Piper lolot.[Pubmed:17941696]
J Agric Food Chem. 2007 Nov 14;55(23):9436-42.
The methanolic extract of Piper lolot, having shown potent inhibitory activity on platelet aggregation induced by arachidonic acid (AA) and platelet activating factor (PAF), was subjected to activity-guided isolation to yield twelve new amide alkaloids, piperlotine A-L (1-12), along with twenty-nine known compounds. Their structures were elucidated on the basis of spectroscopic analysis. The isolated compounds were tested for their inhibitory activity on the rabbit platelet aggregation. The compounds piperlotine A (1), piperlotine C (3), piperlotine D (4), piperlotine E (5), 3-phenyl-1-(2,4,6-trihydroxyphenyl)propan-1-one (21), 3-(4-methoxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one (22), 1-trans-cinnamoylpyrrolidine (24), sarmentine (26), Pellitorine (27), methyl 3-phenylpropionate (32), and (10S)-10-hydroxypheophorbide a methyl ester (40) showed potent antiplatelet aggregation activity.
Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents.[Pubmed:24853616]
Molecules. 2014 May 20;19(5):6428-38.
In the course of our ongoing screening of plants of the family Asteraceae for antiprotozoal activity, a CH2Cl2-extract from the flowering aerial parts of Achillea ptarmica L. (sneezewort yarrow) was found to be active in vitro against Trypanosoma brucei rhodesiense (IC50 = 0.67 microg/mL) and Plasmodium falciparum (IC50 = 6.6 mug/mL). Bioassay guided fractionation led to the isolation and identification of five alkamides from the most active fractions. Pellitorine and 8,9-Z-dehyroPellitorine are the main components of the extract. Beside these olefinic acid amides, four alkamides with diene-diyne structures were isolated. All alkamides were tested for antiprotozoal activity in vitro. Pellitorine was the most active compound so far within this study against P. falciparum (IC50 = 3.3 microg/mL), while 8,9-Z-dehydroPellitorine was most active against T. b. rhodesiense (IC50 = 2.0 microg/mL). The activity of pure Pellitorine against Plasmodium is higher than that of the crude extract and thus explains the activity of the latter. None of the isolated alkamides, however, was as active against T. b. rhodesiense as the crude extract whose antitrypanosomal activity must therfore be due to a synergistic effect of the isolated compounds or to more active yet to be identified constituents.


