MethylproamineDNA-binding radioprotector CAS# 188247-01-0 |
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188247-01-0 | SDF | Download SDF |
| PubChem ID | 448201 | Appearance | Powder |
| Formula | C28H31N7 | M.Wt | 465.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO | ||
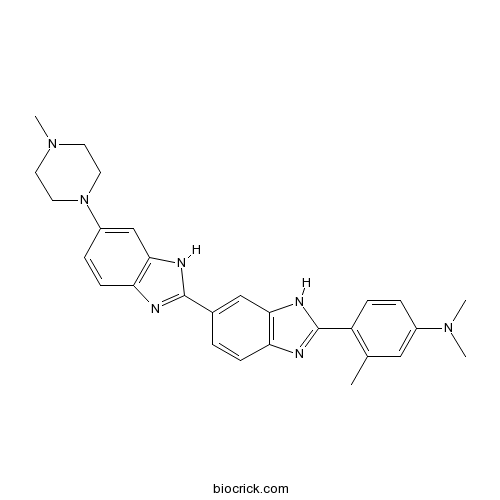
| Chemical Name | N,N,3-trimethyl-4-[6-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-benzimidazol-2-yl]aniline | ||
| SMILES | CC1=C(C=CC(=C1)N(C)C)C2=NC3=C(N2)C=C(C=C3)C4=NC5=C(N4)C=C(C=C5)N6CCN(CC6)C | ||
| Standard InChIKey | ADKLMOJIJGHCCD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H31N7/c1-18-15-20(33(2)3)6-8-22(18)28-30-23-9-5-19(16-25(23)32-28)27-29-24-10-7-21(17-26(24)31-27)35-13-11-34(4)12-14-35/h5-10,15-17H,11-14H2,1-4H3,(H,29,31)(H,30,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methylproamine Dilution Calculator

Methylproamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1478 mL | 10.7391 mL | 21.4781 mL | 42.9562 mL | 53.6953 mL |
| 5 mM | 0.4296 mL | 2.1478 mL | 4.2956 mL | 8.5912 mL | 10.7391 mL |
| 10 mM | 0.2148 mL | 1.0739 mL | 2.1478 mL | 4.2956 mL | 5.3695 mL |
| 50 mM | 0.043 mL | 0.2148 mL | 0.4296 mL | 0.8591 mL | 1.0739 mL |
| 100 mM | 0.0215 mL | 0.1074 mL | 0.2148 mL | 0.4296 mL | 0.537 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Methylproamine is a DNA-binding radioprotector which, on the basis of published pulse radiolysis studies, acts by repair of transient radiation-induced oxidative species on DNA. in vitro: The extent of radioprotection at the clonogenic survival endpoint increased with methylproamine concentration up to a maximum dose modification factor (DMF) of 2.0 at 10 μM. At least 0.1 fmole/nucleus of methylproamine is required to achieve a substantial level of radioprotection (DMF of 1.3) with maximum protection (DMF of 2.0) achieved at 0.23 fmole/nucleus. The γH2AX focus yield per cell nucleus 45 min after irradiation decreased with drug concentration with a DMF of 2.5 at 10 μM [1]. Methylproamine-treated cells had fewer γH2AX foci after IR compared to untreated cells. Also, the presence ofmethylproamine decreased the amount of lower molecular weight DNA entering the gel as shown by the pulsed field gel electrophoresis assay [2]. Experiments with V79 cells have shown that methylproamine is approximately 100-fold more potent than the classical aminothiol radioprotector WR1065. The crystal structures of methylproamine and proamine complexes with the dodecamer d(CGCGAATTCGCG)(2) confirm that the new analogues also are minor groove binders [3]. in vivo: N/A Clinical trial: N/A
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- WKYMVM trifluoroacetate salt
Catalog No.:BCC5815
CAS No.:187986-11-4
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Isodomoic acid G
Catalog No.:BCN1839
CAS No.:188346-81-8
- Pellitorine
Catalog No.:BCN4043
CAS No.:18836-52-7
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
Protection by methylproamine of irradiated human keratinocytes correlates with reduction of DNA damage.[Pubmed:21087168]
Int J Radiat Biol. 2011 Mar;87(3):274-83.
PURPOSE: The therapeutic ratio for ionising radiation treatment of tumour is a trade-off between normal tissue side-effects and tumour control. Application of a radioprotector to normal tissue can reduce side-effects. Here we study the effects of a new radioprotector on the cellular response to radiation. Methylproamine is a DNA-binding radioprotector which, on the basis of published pulse radiolysis studies, acts by repair of transient radiation-induced oxidative species on DNA. To substantiate this hypothesis, we studied protection by Methylproamine at both clonogenic survival and radiation-induced DNA damage, assessed by gammaH2AX (histone 2AX phosphorylation at serine 139) focus formation endpoints. MATERIALS AND METHODS: The human keratinocyte cell line FEP1811 was used to study clonogenic survival and yield of gammaH2AX foci following irradiation ((1)(3)(7)Cs gamma-rays) of cells exposed to various concentrations of Methylproamine. Uptake of Methylproamine into cell nuclei was measured in parallel. RESULTS: The extent of radioprotection at the clonogenic survival endpoint increased with Methylproamine concentration up to a maximum dose modification factor (DMF) of 2.0 at 10 muM. At least 0.1 fmole/nucleus of Methylproamine is required to achieve a substantial level of radioprotection (DMF of 1.3) with maximum protection (DMF of 2.0) achieved at 0.23 fmole/nucleus. The gammaH2AX focus yield per cell nucleus 45 min after irradiation decreased with drug concentration with a DMF of 2.5 at 10 muM. CONCLUSIONS: These results are consistent with the hypothesis that radioprotection by Methylproamine is mediated by attenuation of the extent of initial DNA damage.
Methylproamine protects against ionizing radiation by preventing DNA double-strand breaks.[Pubmed:20732333]
Mutat Res. 2010 Oct 13;692(1-2):49-52.
PURPOSE: The majority of cancer patients will receive radiotherapy (RT), therefore, investigations into advances of this modality are important. Conventional RT dose intensities are limited by adverse responses in normal tissues and a primary goal is to ameliorate adverse normal tissue effects. The aim of these experiments is to further our understanding regarding the mechanism of radioprotection by the DNA minor groove binder, Methylproamine, in a cellular context at the DNA level. MATERIALS AND METHODS: We used immunocytochemical methods to measure the accumulation of phosphorylated H2AX (gammaH2AX) foci following ionizing radiation (IR) in patient-derived lymphoblastoid cells exposed to Methylproamine. Furthermore, we performed pulsed field gel electrophoresis DNA damage and repair assays to directly interrogate the action of Methylproamine on DNA in irradiated cells. RESULTS: We found that Methylproamine-treated cells had fewer gammaH2AX foci after IR compared to untreated cells. Also, the presence of Methylproamine decreased the amount of lower molecular weight DNA entering the gel as shown by the pulsed field gel electrophoresis assay. CONCLUSIONS: These results suggest that Methylproamine acts by preventing the formation of DNA double-strand breaks (dsbs) and support the hypothesis that radioprotection by Methylproamine is mediated, at least in part, by decreasing initial DNA damage.
In vitro studies with methylproamine: a potent new radioprotector.[Pubmed:14871839]
Cancer Res. 2004 Feb 1;64(3):1067-70.
New analogues of the minor groove binding ligand Hoechst 33342 have been investigated in an attempt to improve radioprotective activity. The synthesis, DNA binding, and in vitro radioprotective properties of Methylproamine, the most potent derivative, are reported. Experiments with V79 cells have shown that Methylproamine is approximately 100-fold more potent than the classical aminothiol radioprotector WR1065. The crystal structures of Methylproamine and proamine complexes with the dodecamer d(CGCGAATTCGCG)(2) confirm that the new analogues also are minor groove binders. It is proposed that the DNA-bound Methylproamine ligand acts as a reducing agent by an electron transfer mechanism, repairing transient radiation-induced oxidizing species on DNA.
Radioprotection of targeted and bystander cells by methylproamine.[Pubmed:25245467]
Strahlenther Onkol. 2015 Mar;191(3):248-55.
INTRODUCTION: Radioprotective agents are of interest for application in radiotherapy for cancer and in public health medicine in the context of accidental radiation exposure. Methylproamine is the lead compound of a class of radioprotectors which act as DNA binding anti-oxidants, enabling the repair of transient radiation-induced oxidative DNA lesions. This study tested Methylproamine for the radioprotection of both directly targeted and bystander cells. METHODS: T98G glioma cells were treated with 15 muM Methylproamine and exposed to (137)Cs gamma-ray/X-ray irradiation and He(2+) microbeam irradiation. Radioprotection of directly targeted cells and bystander cells was measured by clonogenic survival or gammaH2AX assay. RESULTS: Radioprotection of directly targeted T98G cells by Methylproamine was observed for (137)Cs gamma-rays and X-rays but not for He(2+) charged particle irradiation. The effect of Methylproamine on the bystander cell population was tested for both X-ray irradiation and He(2+) ion microbeam irradiation. The X-ray bystander experiments were carried out by medium transfer from irradiated to non-irradiated cultures and three experimental designs were tested. Radioprotection was only observed when recipient cells were pretreated with the drug prior to exposure to the conditioned medium. In microbeam bystander experiments targeted and nontargeted cells were co-cultured with continuous Methylproamine treatment during irradiation and postradiation incubation; radioprotection of bystander cells was observed. DISCUSSION AND CONCLUSION: Methylproamine protected targeted cells from DNA damage caused by gamma-ray or X-ray radiation but not He(2+) ion radiation. Protection of bystander cells was independent of the type of radiation which the donor population received.


