Isodomoic acid GCAS# 188346-81-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 188346-81-8 | SDF | Download SDF |
| PubChem ID | 11162706 | Appearance | Powder |
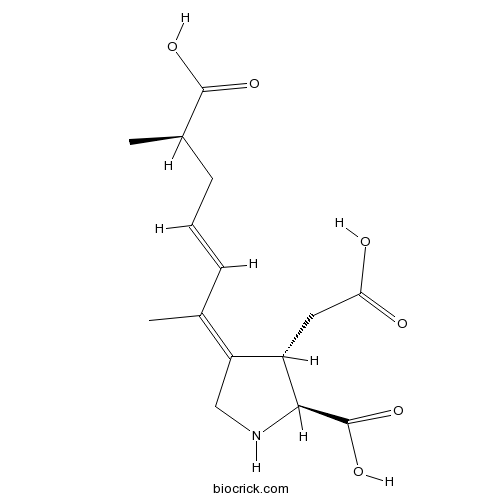
| Formula | C15H21NO6 | M.Wt | 311.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,4E)-4-[(E,6R)-6-carboxyhept-3-en-2-ylidene]-3-(carboxymethyl)pyrrolidine-2-carboxylic acid | ||
| SMILES | CC(CC=CC(=C1CNC(C1CC(=O)O)C(=O)O)C)C(=O)O | ||
| Standard InChIKey | MKCCCBSBSRCARB-NMWGDLGCSA-N | ||
| Standard InChI | InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-4,9-10,13,16H,5-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b4-3+,11-8-/t9-,10+,13+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Total syntheses of isodomoic acids G and H: an exercise in tetrasubstituted alkene synthesis.[Pubmed: 19899794]J Am Chem Soc. 2009 Dec 9;131(48):17714-8.
|
| Structure Identification | Org Lett. 2003 Oct 2;5(20):3771-3.First total synthesis and stereochemical definition of isodomoic acid G.[Pubmed: 14507227]
J Org Chem. 2011 Jan 7;76(1):201-15.Stereocontrolled total syntheses of isodomoic acids G and H via a unified strategy.[Pubmed: 21121685]Marine neuroexcitatory compounds Isodomoic acid G and isodomoic acid H were efficiently synthesized from a common intermediate using a silicon-based cross-coupling reaction.
|

Isodomoic acid G Dilution Calculator

Isodomoic acid G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.212 mL | 16.0601 mL | 32.1203 mL | 64.2405 mL | 80.3006 mL |
| 5 mM | 0.6424 mL | 3.212 mL | 6.4241 mL | 12.8481 mL | 16.0601 mL |
| 10 mM | 0.3212 mL | 1.606 mL | 3.212 mL | 6.4241 mL | 8.0301 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6424 mL | 1.2848 mL | 1.606 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6424 mL | 0.803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Massonianoside B
Catalog No.:BCN1164
CAS No.:188300-19-8
- Californidine
Catalog No.:BCC8137
CAS No.:18830-99-4
- 8alpha-(2-Methylacryloyloxy)hirsutinolide
Catalog No.:BCN7109
CAS No.:188293-70-1
- (±)-Propionylcarnitine chloride
Catalog No.:BCC6719
CAS No.:18828-58-5
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Pellitorine
Catalog No.:BCN4043
CAS No.:18836-52-7
- MAFP
Catalog No.:BCC7059
CAS No.:188404-10-6
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
Stereocontrolled total syntheses of isodomoic acids G and H via a unified strategy.[Pubmed:21121685]
J Org Chem. 2011 Jan 7;76(1):201-15.
Marine neuroexcitatory compounds isodomoic acids G and H were efficiently synthesized from a common intermediate using a silicon-based cross-coupling reaction. Dividing each target compound into the core fragment and the side-chain fragment enabled the synthesis to be convergent. The trans-2,3-disubstituted pyrrolidine core fragment was accessed through a diastereoselective rhodium-catalyzed carbonylative silylcarbocyclization reaction of a vinylglycine-derived 1,6-enyne. A stereochemically divergent desilylative iodination reaction was developed to convert the cyclization product to both E- and Z-alkenyl iodides, which would eventually lead to Isodomoic acid G and isodomoic acid H, respectively. The late-stage alkenyl-alkenyl silicon-based cross-coupling reaction uniting the core alkenyl iodides and the side-chain alkenylsilanol was achieved under mild conditions. Finally, two mild deprotections afforded the target molecules.
First total synthesis and stereochemical definition of isodomoic acid G.[Pubmed:14507227]
Org Lett. 2003 Oct 2;5(20):3771-3.
[structure: see text] The first total synthesis and stereochemical definition of Isodomoic acid G has been achieved. The key nickel-catalyzed coupling of an alkynyl enone with an alkenylzirconium allows formation of the pyrrolidine ring and most of the stereochemical features in a single step. This report provides the first total synthesis application of this new reaction and illustrates its utility in the stereoselective preparation of highly substituted 1,3-dienes.
Total syntheses of isodomoic acids G and H: an exercise in tetrasubstituted alkene synthesis.[Pubmed:19899794]
J Am Chem Soc. 2009 Dec 9;131(48):17714-8.
A unified approach to the pyrrolidine triacid natural products isodomoic acids G and H has been developed. Total syntheses of both natural products were completed, and determination of the correct stereostructure of Isodomoic acid G was established by comparing 5'-(R) and 5'-(S) isomers to a sample of authentic material. A nickel-catalyzed cyclization constructs the pyrrolidine ring while simultaneously establishing either the E or Z stereochemistry of an exocyclic tetrasubstituted alkene. Stereoselective assembly of both the E- and Z-alkenes of the natural products is made possible by a predictable strategy that alters the timing of substituent introduction to control alkene stereochemistry.


