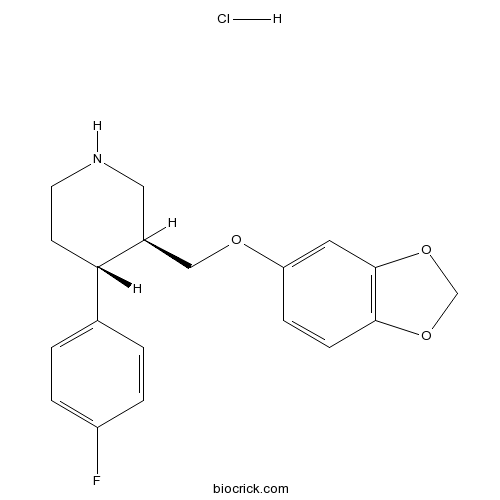
Paroxetine HClAntidepressant agents CAS# 78246-49-8 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78246-49-8 | SDF | Download SDF |
| PubChem ID | 62878 | Appearance | Powder |
| Formula | C19H21ClFNO3 | M.Wt | 365.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BRL29060 hydrochloride; BRL29060A | ||
| Solubility | DMSO : 100 mg/mL (273.35 mM; Need ultrasonic) H2O : 5 mg/mL (13.67 mM; Need ultrasonic) | ||
| Chemical Name | (3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine;hydrochloride | ||
| SMILES | C1CNCC(C1C2=CC=C(C=C2)F)COC3=CC4=C(C=C3)OCO4.Cl | ||
| Standard InChIKey | GELRVIPPMNMYGS-RVXRQPKJSA-N | ||
| Standard InChI | InChI=1S/C19H20FNO3.ClH/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18;/h1-6,9,14,17,21H,7-8,10-12H2;1H/t14-,17-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Paroxetine hydrochloride is a potent selective serotonin-reuptake inhibitor, commonly prescribed as an antidepressant and has GRK2 inhibitory ability with IC50 of 14 μM.In Vitro:Paroxetine (1 μM and 10 μM) distinctly restrains T cell migration induced by CX3CL1 through inhibiting GRK2. Paroxetine inhibits GRK2 induced activation of ERK[1]. Paroxetine (10 μM) reduces pro-inflammatory cytokines in LPS-stimulated BV2 cells. Paroxetine (0-5 μM) leads to a dose-dependent inhibition on LPS-induced production of TNF-α and IL-1β in BV2 cells. Paroxetine also inhibits lipopolysaccharide (LPS)-induced nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression in BV2 cells. Paroxetine (5 μM) blocks LPS-induced JNK activation and attenuates baseline ERK1/2 activity in BV2 cells. Paroxetine relieves microglia-mediated neurotoxicity, and suppresses LPS-stimulated pro-inflammatory cytokines and NO in primary microglial cells[4].In Vivo:Paroxetine treatment obviously attenuates the symptoms of CIA rats. Paroxetine treatment clearly prevents the histological damage of joints and alleviates T cells infiltration into synovial tissue. Paroxetine reveals a strong effect on inhibiting CX3CL1 production in synovial tissues[1]. Paroxetine (20 mg/kg/day) reduces the myocyte cross-sectional area in rat and ROS formation in the remote myocardium. Paroxetine reduces the susceptibility to ventricular tachycardia. Paroxetine treatment following MI decreases LV remodeling and susceptibility to arrhythmias, probably by reducing ROS formation[2]. In CCI paroxetine-treated group, paroxetine (10 mg/kg, i.p.) produces hyperalgesia at days 7 and 10 (P<0.01), but a decrease in pain behavior is seen at day 14. Moreover, paroxetine (10 mg/kg) significantly attenuates tactile hypersensitivity when compared to CCI vehicle-treated group[5]. References: | |||||

Paroxetine HCl Dilution Calculator

Paroxetine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7335 mL | 13.6676 mL | 27.3351 mL | 54.6702 mL | 68.3378 mL |
| 5 mM | 0.5467 mL | 2.7335 mL | 5.467 mL | 10.934 mL | 13.6676 mL |
| 10 mM | 0.2734 mL | 1.3668 mL | 2.7335 mL | 5.467 mL | 6.8338 mL |
| 50 mM | 0.0547 mL | 0.2734 mL | 0.5467 mL | 1.0934 mL | 1.3668 mL |
| 100 mM | 0.0273 mL | 0.1367 mL | 0.2734 mL | 0.5467 mL | 0.6834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Paroxetine Hydrochloride is a antidepressant agents known as selective serotonin-reuptake inhibitors (SSRIs).Paroxetine is a potent and highly selective inhibitor of neuronal serotonin reuptake. Paroxetine likely inhibits the reuptake of serotonin at the
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
- Ecliptasaponin A
Catalog No.:BCN3843
CAS No.:78285-90-2
- 7-Ethylcamptothecin
Catalog No.:BCN2480
CAS No.:78287-27-1
- H-D-1-Nal-OH
Catalog No.:BCC3281
CAS No.:78306-92-0
- MRK 016
Catalog No.:BCC6070
CAS No.:783331-24-8
- MLN120B
Catalog No.:BCC1772
CAS No.:783348-36-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- Nocamycin I
Catalog No.:BCN1845
CAS No.:78339-49-8
- Pyranojacareubin
Catalog No.:BCN7429
CAS No.:78343-62-1
- MY-5445
Catalog No.:BCC6645
CAS No.:78351-75-4
Generation of hydrate forms of paroxetine HCl from the amorphous state: an evaluation of thermodynamic and experimental predictive approaches.[Pubmed:25592956]
Int J Pharm. 2015 Mar 15;481(1-2):114-24.
In this study, we evaluate the use of theoretical thermodynamic analysis of amorphous paroxetine hydrochloride (HCl) as well as experimental assessment in order to identify the most promising approach to stability and dissolution behaviour prediction, particularly in relation to stoichiometric and nonstoichiometric hydrate formation. Differential scanning calorimetry, thermogravimetric analysis, Fourier transform infrared and X-ray diffraction techniques were used. Parameters including heat capacity, configurational thermodynamic quantities, fragility and relaxation time classified amorphous Paroxetine HCl as a moderate fragile glass with a considerable degree of molecular mobility. Solubility studies indicated little advantage of the amorphous form over the crystalline due to conversion to the hydrate Form I during equilibration, while the dissolution rate was higher for the amorphous form under sink conditions. A marked difference in the physical stability of amorphous Paroxetine HCl was observed between dry and low humidity storage, with the system recrystallizing to the hydrate form. We conclude that, in this particular case (amorphous conversion to the hydrate), water may be playing a dual role in both plasticizing the amorphous form and driving the equilibrium towards the hydrate form, hence prediction of recrystallization behaviour from amorphous characteristics may be confounded by the additional process of hydrate generation.
Spectrophotometric Determination of the Antidepressants Sertraline and Paroxetine HCl using 2,4-Dinitrofluorobenzene.[Pubmed:23675200]
Int J Biomed Sci. 2010 Sep;6(3):252-9.
A simple and sensitive spectrophotometric method was developed for the determination of each of sertraline (SER) and Paroxetine HCl (PXT) in dosage forms. The method is based upon reaction of PXT and SER with 2,4-dinitrofluorobenzene (DNFB) to form colored products. The absorbance of the products were measured at 375and 390 nm for SER and PXT respectively. The absorbance concentration plots were rectilinear over the concentration rang of 1-10 and 2-20 mug/mL with lower detection limits (LOD) of 0.11 and 0.28 mug/mL and quantification limits (LOQ) of 0.32 and 0.85 mug/mL for SER and PXT, respectively. The developed method was successfully applied for the determination of SER and PXT in dosage forms. The common excipients and additives did not interfere in their determinations. There was no significant difference between the results obtained by the proposed and the reference methods regarding Student t-test and the variance ratio F-test respectively. A proposal of the reaction pathway was postulated.
Development and validation of a method for the analysis of paroxetine HCl by circular dichroism.[Pubmed:23532996]
Chirality. 2013 Apr;25(4):211-4.
A simple, rapid, and sensitive method for the analysis of paroxetine, in tablets as well as the pure drug, by circular dichroism is described. The method was validated for repeatability, linearity, limit of detection, limit of quantification, and recovery according to the International Conference on Harmonization guidelines. Excellent results were obtained, within the globally accepted validation reference values, particularly taking into account the low concentration levels investigated. This is the first report of the quantitation of paroxetine, a chiral drug, without previous separation of the analyte. Additionally, the solid state CD spectrum of PXT was obtained.
Identification and characterization of stoichiometric and nonstoichiometric hydrate forms of paroxetine HCl: reversible changes in crystal dimensions as a function of water absorption.[Pubmed:23051151]
Mol Pharm. 2012 Dec 3;9(12):3515-25.
Paroxetine hydrochloride (HCl) is an antidepressant drug, reported to exist in the anhydrous form (form II) and as a stable hemihydrate (form I). In this study, we investigate the hydration behavior of Paroxetine HCl form II with a view to understanding both the nature of the interaction with water and the interchange between forms II and I as a function of both temperature and water content. In particular, we present new evidence for both the structure and the interconversion process to be more complex than previously recognized. A combination of characterization techniques was used, including thermal (differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA)), spectroscopic (attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR)), dynamic vapor sorption (DVS) and X-ray powder diffraction (XRPD) with variable humidity, along with computational molecular modeling of the crystal structures. The total amount of water present in form II was surprisingly high (3.8% w/w, 0.8 mol of water/mol of drug), with conversion to the hemihydrate noted on heating in hermetically sealed DSC pans. XRPD, supported by ATR-FTIR and DVS, indicated changes in the unit cell dimensions as a function of water content, with clear evidence for reversible expansion and contraction as a function of relative humidity (RH). Based on these data, we suggest that Paroxetine HCl form II is not an anhydrate but rather a nonstoichiometric hydrate. However, no continuous channels are present and, according to molecular modeling simulation, the water is moderately strongly bonded to the crystal, which is in itself an uncommon feature when referring to nonstoichiometric hydrates. Overall, therefore, we suggest that the anhydrous form of Paroxetine HCl is not only a nonstoichiometric hydrate but also one that shows highly unusual characteristics in terms of gradual unit cell expansion and contraction despite the absence of continuous channels. These structural features in turn influence the tendency of this drug to convert to the more stable hemihydrate. The study has implications for the recognition and understanding of the behavior of pharmaceutical nonstoichiometric hydrates.


