Octanoic acidCAS# 124-07-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124-07-2 | SDF | Download SDF |
| PubChem ID | 379 | Appearance | Oil |
| Formula | C8H16O2 | M.Wt | 144.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | octanoic acid | ||
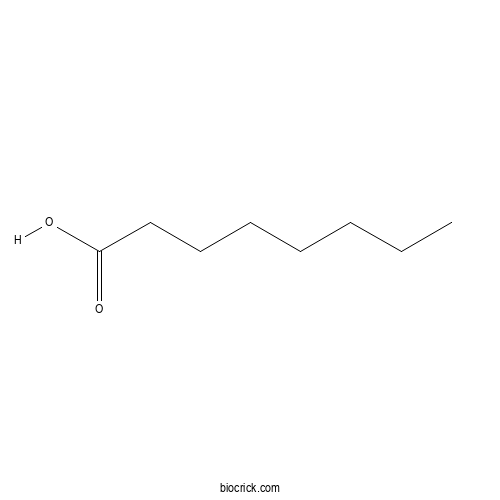
| SMILES | CCCCCCCC(=O)O | ||
| Standard InChIKey | WWZKQHOCKIZLMA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Octanoic acid is toxic for many insect species. | |||||

Octanoic acid Dilution Calculator

Octanoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9348 mL | 34.6741 mL | 69.3481 mL | 138.6963 mL | 173.3703 mL |
| 5 mM | 1.387 mL | 6.9348 mL | 13.8696 mL | 27.7393 mL | 34.6741 mL |
| 10 mM | 0.6935 mL | 3.4674 mL | 6.9348 mL | 13.8696 mL | 17.337 mL |
| 50 mM | 0.1387 mL | 0.6935 mL | 1.387 mL | 2.7739 mL | 3.4674 mL |
| 100 mM | 0.0693 mL | 0.3467 mL | 0.6935 mL | 1.387 mL | 1.7337 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- 5-Methoxyflavanone
Catalog No.:BCN9755
CAS No.:55947-36-9
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
- Rhoeadine
Catalog No.:BCN9760
CAS No.:2718-25-4
- Quinine sulfate dihydrate
Catalog No.:BCN9761
CAS No.:6119-70-6
- 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one
Catalog No.:BCN9762
CAS No.:487-26-3
- Dodeca 2E,4E,8Z,10E,Z-tetraenoic acid isobutylamide
Catalog No.:BCN9763
CAS No.:866602-52-0
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- (S)-(-)-Limonene
Catalog No.:BCN9766
CAS No.:5989-54-8
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
Characterization of complexes formed between debranched starch and fatty acids having different carbon chain lengths.[Pubmed:33278451]
Int J Biol Macromol. 2020 Dec 2. pii: S0141-8130(20)35136-9.
Recently, amylose-lipid complexes have attracted widespread attention because of their various applications. However, DBS complexed with fatty acids of different carbon chain length are rarely studied. This study aimed to probe the complexation of DBS with saturated fatty acids having different carbon chain lengths (C6-C18). The results revealed that DBS was able to form V-type complexes with all the fatty acids considered. Compared to DBS, the relative crystallinity of the complexes increased 2-3 times. DBS with lauric acid and myristic acid formed three types V-type complexes (type I, type IIa, and type IIb). The complexing index followed the order of hexanoic acid > Octanoic acid > capric acid > lauric acid > myristic acid > palmitic acid > stearic acid. Furthermore, lauric acid and myristic acid formed complexes with DBS more easily compared with other fatty acids.
Vitamin D3 bioaccessibility: Influence of fatty acid chain length, salt concentration and l-alpha-phosphatidylcholine concentration on mixed micelle formation and delivery of vitamin D3.[Pubmed:33277128]
Food Chem. 2020 Nov 25:128722.
Vitamin D (VD) is a fat-soluble vitamin with high deficiency levels evident globally. Bioaccessibility of VD is influenced by formation of mixed micelles (MM) during digestion. This study assessed the impact of fatty acid (FA) type, phospholipid concentration on MM formation and stability of MM to salts. MM formation occurred at NaCl and KCl concentrations ranging from 20 to 100 mM, when Octanoic acid (C8) or stearic acid (C18) were used. MM hydrodynamic size increased with increasing l-alpha-phosphatidylcholine concentration (1.5-7.5 mM) for both C8 and C18, above which concentration MM did not form. FA chain length impacted MM with hydrodynamic size increasing from 3.8 nm for decanoic acid (C10) to 4.4 nm for C18. VD3 incorporation in MM was not influenced by the FA used (C10 or C18). Understanding stability and formation of MM and VD3 loading is an essential first step towards manipulating food structures for improving delivery of VD.
Joint Hypermobility Syndrome in Patients With Functional Dyspepsia.[Pubmed:33259162]
Clin Transl Gastroenterol. 2020 Nov;11(11):e00220.
INTRODUCTION: The pathophysiology underlying functional dyspepsia (FD) is multifactorial and focuses on gastric sensorimotor dysfunction. Recent studies demonstrated that joint hypermobility syndrome (JHS) is strongly associated with unexplained dyspeptic symptoms in patients attending gastrointestinal clinics. We aimed to study the relationship between symptoms, gastric sensorimotor function, and JHS in FD patients. METHODS: Tertiary care FD patients who underwent a gastric barostat study and a gastric emptying breath test with 13C-Octanoic acid were recruited for assessment of JHS. The presence of JHS was evaluated by a 2-phase interview and clinical examination that included major and minor criteria of the Brighton classification. RESULTS: A total of 62 FD patients (68% women, age 44 +/- 1.8 years, and body mass index: 21.7 +/- 0.7 kg/m) accepted to participate in the study. JHS was diagnosed in 55% of FD patients. Assessed symptom profiles during the visit did not differ between the groups. Delayed gastric emptying was not significantly more common in JHS group compared with non-JHS group (JHS group 32% vs non-JHS group 16%, P = 0.31). Prevalence of hypersensitivity to distention (JHS group 24% vs non-JHS group 29%, P = 0.76) and impaired gastric accommodation (JHS group 38% vs non-JHS group 42%, P = 0.79) was similar in patients with or without JHS. No correlations were found between the Beighton hypermobility score and gastric compliance (r = 0.09). DISCUSSION: A large subset of this study cohort of tertiary care FD patients has coexisting JHS. We did not identify any specific differences in gastric sensorimotor function between patients with and without JHS. Further prospective research will be required to elucidate the relationship between JHS, a multisystemic disorder with widespread manifestations, and FD symptoms.
Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol.[Pubmed:33252828]
Microb Biotechnol. 2020 Nov 30.
Candida biofilms are tolerant to conventional antifungal therapeutics and the host immune system. The transition of yeast cells to hyphae is considered a key step in C. albicans biofilm development, and this transition is inhibited by the quorum-sensing molecule farnesol. We hypothesized that fatty acids mimicking farnesol might influence hyphal and biofilm formation by C. albicans. Among 31 saturated and unsaturated fatty acids, six medium-chain saturated fatty acids, that is, heptanoic acid, Octanoic acid, nonanoic acid, decanoic acid, undecanoic acid and lauric acid, effectively inhibited C. albicans biofilm formation by more than 75% at 2 microg ml(-1) with MICs in the range 100-200 microg ml(-1) . These six fatty acids at 2 microg ml(-1) and farnesol at 100 microg ml(-1) inhibited hyphal growth and cell aggregation. The addition of fatty acids to C. albicans cultures decreased the productions of farnesol and sterols. Furthermore, down-regulation of several hyphal and biofilm-related genes caused by heptanoic or nonanoic acid closely resembled the changes caused by farnesol. In addition, nonanoic acid, the most effective compound diminished C. albicans virulence in a Caenorhabditis elegans model. Our results suggest that medium-chain fatty acids inhibit more effectively hyphal growth and biofilm formation than farnesol.
Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties.[Pubmed:33234437]
Food Chem. 2020 Nov 13:128603.
Enzymatic lipophilization is an important process to extend the use of anthocyanins in lipidic media. In this work delphinidin 3-O-sambubioside (Dp3sam) isolated from Hibiscus sabdariffa L. flower was esterified with Octanoic acid using Candida antarctica lipase B. The physical-chemical properties of the new lipophilic pigment were studied by UV-vis spectroscopy. Dp3sam with chloride, acetate and formate as counter ions were employed to study the lipophilization reaction. The hydrolysis of the reagent was avoided with a formate counter ion and the expected product was achieved with a noteworthy change of solubility. 1D and 2D NMR characterization of Dp3sam-C8 confirmed that the lipophilization took place at the primary alcohol of the glucoside moiety. Overall, the Dp3sam-C8 ester presents a stabilization of the quinoidal base (blue color) at neutral or moderate alkaline pH, which foresees a potential use of this pigment as a broad kind of industries on lipo-soluble formulations.
Changes in Fatty Acid and Volatile Compound Profiles during Storage of Smoked Cheese Made from the Milk of Native Polish Cow Breeds Raised in the Low Beskids.[Pubmed:33198354]
Animals (Basel). 2020 Nov 12;10(11). pii: ani10112103.
This study investigated changes in the proximate chemical composition and profiles of fatty acids and volatile compounds of 12 smoked cheeses made from the milk of native Polish cow breeds used in Beskid Niski. Analyses were performed during the shelf life i.e., in the 1st, 21st, 42nd and 69th day of storage. Studies have shown that thanks to smoking and vacuum-packing, the chemical composition of cheese remained stable throughout the whole shelf-life. Up until the 21st day of storage, there were no statistically significant changes in the profile of fatty acids as well as volatile compounds. Changes were observed only after the mentioned storage time. After 21 days, there was a significant (p < 0.05) and steady decrease (up to day 69) in the proportion of odd-chain (by about 36%), branched-chain (by about 17%) and unsaturated fatty acids (by slightly over 1%). Among unsaturated fatty acids (p < 0.05), however, there was a significant increase in the proportion of monounsaturated fatty acids (by 5%) and a decrease in polyunsaturated fatty acids of nearly 12%. Storage lowered (by 47% in the 69th day of storage) the content of the conjugated linoleic acids (CLA), as well as lowered the n6 to n3 fatty acids ratio. During the 69 days of storage, the content of carboxylic acids increased to more than 50%. In the period from the 42nd to 69th day of cheese storage, the content of butyric acid and hexanoic acids increased twofold, whereas that of Octanoic acid increased more than tenfold. Fifty-four volatile compounds were identified in the cheese. The largest group was ketones (34%), whose level decreased during storage, with 2-butanone, 3-hydroxy- (acetoin) and 2-butanone predominating. The research found that due to their low odor threshold, carboxylic acids may have negatively affected the flavor profile of the cheese.
What is specific in adsorption of perfluoroalkyl acids on carbon materials?[Pubmed:33121799]
Chemosphere. 2020 Oct 5:128520.
Various activated carbon products show wide variability in adsorption performance towards perfluoroalkyl acids (PFAAs) and predictive tools are largely missing. In order to gain a better understanding on the adsorption mechanisms of PFAAs, perfluoroOctanoic acid (PFOA) was compared with its fluorine-free analogon Octanoic acid (OCA) as well as phenanthrene (nonionic) in terms of their response towards changes in carbon surface chemistry. For this approach, a commercial activated carbon felt (ACF) with high content of acidic surface groups was modified by amino-functionalisation as well as thermal defunctionalisation in H2 (yielding DeCACF). While improvement by amino-functionalisation was moderate, defunctionalisation drastically enhanced adsorption of PFOA and other PFAAs. In comparison, OCA and phenanthrene were much less affected. Electrostatic interactions and charge compensation provided by positively charged surface sites (quantified by their anion exchange capacity) are obviously more crucial for PFAAs than for common organic acids (such as the tested OCA). A possible reason is their exceptionally strong acidity with pKa < 1. Nevertheless, at the best modified ACF material (DeCACF) the sorption coefficients (Kd) for PFOA and perfluorooctylsulfonic acid (PFOS) at environmentally relevant concentrations reach the range of 10(7) L/kg which is outstanding. DeCACF provides a surface with overall low polarity (low O-content), low density of acidic sites causing electrostatic repulsion, but nevertheless a sufficient density of charge-balancing sites for organic anions. The results of the present study contribute to an optimized selection of adsorbents for PFAA adsorption from water considering also various salt matrices and the presence of natural organic matter.
Effects of a Small Increase in Carbon Dioxide Pressure during Fermentation on Wine Aroma.[Pubmed:33086729]
Foods. 2020 Oct 19;9(10). pii: foods9101496.
The present study tested the effect of a slight increase in pressure (from 0 to 1 bar) during the fermentation on the wine aroma profile. Fermentations were carried out with a commercial dry yeast on Sangiovese juice in the absence of berry skins. The wine samples fermented under slight overpressure conditions were found to be significantly different from the control samples produced at atmospheric pressure in relation to several chemical compounds. Concentrations of many esters (i.e., isoamyl acetate, ethyl acetate, ethyl hexanoate, hexyl acetate, ethyl dodecanoate, and ethyl tetradecanoate), and acids (i.e., hexanoic acid, Octanoic acid, and decanoic acid) increased, while concentrations of two acids (i.e., isobutyric and isovaleric acid) decreased. These differences, notably the higher concentration of esters, are usually associated with a more intense fruity attribute. Triangular sensory tests revealed that the significant chemical differences were also perceivable; hence, introducing a slight pressure increase during the alcoholic fermentation could be a useful tool in managing the aroma profile of wine.
Dynamic Metabolome Analysis Reveals the Metabolic Fate of Medium-Chain Fatty Acids in AML12 Cells.[Pubmed:33073987]
J Agric Food Chem. 2020 Oct 28;68(43):11997-12010.
Several studies in hepatocyte cell lines reported that medium-chain fatty acids (MCFAs) with 6-12 carbons showed different metabolic properties from long-chain fatty acids (LCFAs). However, these studies reported unclear effects of different fatty acid molecules on hepatocyte metabolism. This study is aimed to capture the metabolic kinetics of MCFA assimilation in AML12 cells treated with Octanoic acid (FA 8:0), decanoic acid (FA 10:0), or lauric acid (FA12:0) [LCFA; oleic acid (FA 18:1)] via metabolic profiling and dynamic metabolome analysis with (13)C-labeling. The concentrations of total ketone bodies in the media of cells treated with FA 8:0 or FA 10:0 were 3.22- or 3.69-fold higher than those obtained with FA 18:1 treatment, respectively. FA 12:0 treatment did not significantly increase ketone body levels compared to DMSO treatment (control), whereas FA 12:0 treatment increased intracellular triacylglycerol (TG) levels 15.4 times compared to the control. Metabolic profiles of FA 12:0-treated samples differed from those of the FA 8:0-treated and FA 10:0-treated samples, suggesting that metabolic assimilation of MCFAs differed significantly depending on the MCFA type. Furthermore, the dynamic metabolome analysis clearly revealed that FA 8:0 was rapidly and quantitatively oxidized to acetyl-CoA and assimilated into ketone bodies, citrate cycle intermediates, and glucogenic amino acids but not readily into TGs.
Characterization of the key aroma compounds in Yunnan goat milk cake using a sensory-directed flavor analysis.[Pubmed:33063315]
J Food Sci. 2020 Nov;85(11):3981-3997.
To identify the key aroma compounds in Yunnan goat milk cake, seven varieties of milk cake samples were subjected to sensory analysis and gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), aroma recombination, omission, and addition tests. The GC-MS results revealed 53 compounds with aroma characteristics in all the samples. A further comparison of odor activity values and aroma intensities (AI) revealed 25 of these compounds as the initial key aroma compounds. The contributions of these key aroma compounds to the sensory attributes were determined using a partial least squares regression. Of these compounds, 2-heptanone and 2-nonanone were closely related to the "milky" and "cheesy" attributes and were highly abundant in the samples from Kunming. Fatty acids, including butanoic acid, hexanoic acid, Octanoic acid, and decanoic acid, were the most abundant compounds detected in the milk cakes. These fatty acids were closely related to the "rancid" and "animalic (goat)" attributes and were largely detected in the samples from Dali Dengchuan and Dali Xiaguan. Sensory-directed aroma recombination, omission, and addition tests further validated the important contributions of ethyl butyrate, benzaldehyde, 3-methyl-1-butanol, 2-heptanone, hexanoic acid, and Octanoic acid to the overall sensory properties. Moreover, ethyl butyrate, benzaldehyde, and 2-heptanone, when added, had evident inhibitory or masking effects on the AI of "sour," "rancid," and "animalic (goat)" attributes. PRACTICAL APPLICATION: Goat milk cake is a popular acid-curd cheese in Yunnan, China, however, our limited knowledge to its key aroma compounds restricts its development and industrial production. In this study, a sensory-directed flavor analysis was used to characterized the key aroma compounds of Yunnan goat milk cake, which will help to enhance our understanding on the flavor profile of Yunnan goat milk cake and provide a reference for optimizing the flavor feature and organoleptic quality of this fresh goat cheese.
Sensory Perception of Non-Deuterated and Deuterated Organic Compounds.[Pubmed:33058253]
Chemistry. 2020 Oct 15.
The chemical background of olfactory perception has been subject of intensive research, but no available model can fully explain the sense of smell. There are also inconsistent results on the role of the isotopology of molecules. In experiments with human subjects it was found that the isotope effect is weak with acetone and D6 -acetone. In contrast, clear differences were observed in the perception of Octanoic acid and D15 -Octanoic acid. Furthermore, a trained sniffer dog was initially able to distinguish between these isotopologues of Octanoic acid. In chromatographic measurements, the respective deuterated molecule showed weaker interaction with a non-polar liquid phase. Quantum chemical calculations give evidence that deuterated Octanoic acid binds more strongly to a model receptor than non-deuterated. In contrast, the binding of the non-deuterated molecule is stronger with acetone. The isotope effect is calculated in the framework of statistical mechanics. It results from a complicated interplay between various thermostatistical contributions to the non-covalent free binding energies and it turns out to be very molecule-specific. The vibrational terms including non-classical zero-point energies play about the same role as rotational/translational contributions and are larger than bond length effects for the differential isotope perception of odor for which general rules cannot be derived.
Transformation of Microbial Negative Correlations into Positive Correlations by Saccharomyces cerevisiae Inoculation during Pomegranate Wine Fermentation.[Pubmed:33036987]
Appl Environ Microbiol. 2020 Nov 24;86(24). pii: AEM.01847-20.
The application of starter is a common practice to accelerate and steer the pomegranate wine fermentation process. However, the use of starter needs a better understanding of the effect of the interaction between the starter and native microorganisms during alcoholic fermentation. In this study, high-throughput sequencing combined with metabolite analysis was applied to analyze the effect of commercial Saccharomyces cerevisiae inoculation on the native fungal community interaction and metabolism during pomegranate wine fermentation. Results showed that there were diverse native fungi in pomegranate juice, including Hanseniaspora uvarum, Hanseniaspora valbyensis, S. cerevisiae, Pichia terricola, and Candida diversa Based on ecological network analysis, we found that S. cerevisiae inoculation transformed the negative correlations into positive correlations among the native fungal communities and decreased the Granger causalities between native yeasts and volatile organic compounds. This might lead to decreased contents of isobutanol, isoamylol, Octanoic acid, decanoic acid, ethyl laurate, ethyl acetate, ethyl hexadecanoate, phenethyl acetate, and 2-phenylethanol during fermentation. This study combined correlation and causality analysis to gain a more integrated understanding of microbial interaction and the fermentation process. It provided a new strategy to predict certain behaviors between inoculated and selected microorganisms and those coming directly from the fruit.IMPORTANCE Microbial interactions play an important role in flavor metabolism during traditional food and beverage fermentation. However, we understand little about how selected starters influence interactions among native microorganisms. In this study, we found that S. cerevisiae inoculation changed the interactions and metabolisms of native fungal communities during pomegranate wine fermentation. This study not only suggests that starter inoculation should take into account the positive features of starters but also characterizes the microbial interactions established among the starters and the native communities. It may be helpful to select appropriate starter cultures for winemakers to design different styles of wine.
Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA).[Pubmed:33033332]
Sci Rep. 2020 Oct 8;10(1):16789.
Perfluoroalkyl substances (PFAS) are a class of compounds used in industry and consumer products. PerfluoroOctanoic acid (PFOA) is the predominant form in human samples and has been shown to induce severe health consequences, such as neonatal mortality, neurotoxicity, and immunotoxicity. Toxicological studies indicate that PFAS accumulate in bone tissues and cause altered bone development. Epidemiological studies have reported an inverse relationship between PFAS and bone health, however the associated mechanisms are still unexplored. Here, we present computational, in silico and in vitro evidence supporting the interference of PFOA on vitamin D (VD). First, PFOA competes with calcitriol on the same binding site of the VD receptor, leading to an alteration of the structural flexibility and a 10% reduction by surface plasmon resonance analysis. Second, this interference leads to an altered response of VD-responsive genes in two cellular targets of this hormone, osteoblasts and epithelial cells of the colorectal tract. Third, mineralization in human osteoblasts is reduced upon coincubation of PFOA with VD. Finally, in a small cohort of young healthy men, PTH levels were higher in the exposed group, but VD levels were comparable. Altogether these results provide the first evidence of endocrine disruption by PFOA on VD pathway by competition on its receptor and subsequent inhibition of VD-responsive genes in target cells.
An effective chromatographic fingerprinting workflow based on comprehensive two-dimensional gas chromatography - Mass spectrometry to establish volatiles patterns discriminative of spoiled hazelnuts (Corylus avellana L.).[Pubmed:33011466]
Food Chem. 2021 Mar 15;340:128135.
The volatile fraction of hazelnuts encrypts information about: cultivar/geographical origin, post-harvest treatments, oxidative stability and sensory quality. However, sensory features could be buried under other dominant chemical signatures posing challenges to an effective classification based on pleasant/unpleasant notes. Here a novel workflow that combines Untargeted and Targeted (UT) fingerprinting on comprehensive two-dimensional gas-chromatographic patterns is developed to discriminate spoiled hazelnuts from those of acceptable quality. By flash-profiling, six hazelnut classes are defined: Mould, Mould-rancid-solvent, Rancid, Rancid-stale, Rancid-solvent, and Uncoded KO. Chromatographic fingerprinting on composite 2D chromatograms from samples belonging to the same class (i.e., composite class-images) enabled effective selection of chemical markers: (a) Octanoic acid that guides the sensory classification being positively correlated to mould; (b) y-nonalactone, y-hexalactone, acetone, and 1-nonanol that are decisive to classify OK and rancid samples; (c) heptanoic and hexanoic acids and y-octalactone present in high relative abundance in rancid-solvent and rancid-stale samples.
Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles.[Pubmed:33007693]
Food Chem. 2021 Mar 15;340:128179.
This study is sought to identify the components in greengage wine that predict the sensory properties. Taste-active compounds and aroma-active compounds of 20 commercially available greengage wines from different regions were characterized. The relationship between these compounds, wine samples and sensory attributes was modeled by partial least squares regression. The regression analysis indicated the taste-active compounds, alanine, leucine, proline, glutamic acid, lysine, malic acid, citric acid, sucrose, glucose, gallic acid, caffeic acid and tannin made a great contribution to the characteristic taste or mouthfeel of greengage wine. Meanwhile, the aroma-active compounds, including ethyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, 3-methylbutanol, 5-hydroxymethylfurfural, Octanoic acid and benzaldehyde, modeled well with the flavor characteristic of greengage wine. The study revealed new insights into the relationship between chemistry and wine sensory characters, which has implications for developing an objective measurement system for determining greengage wine quality.


