2'-MethoxyflavoneCAS# 19725-47-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19725-47-4 | SDF | Download SDF |
| PubChem ID | 146492 | Appearance | Powder |
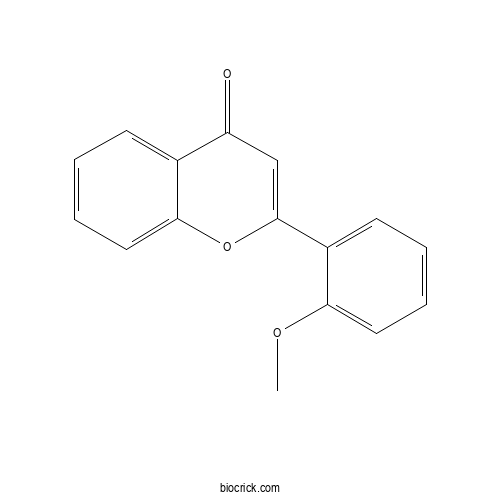
| Formula | C16H12O3 | M.Wt | 252.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(2-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=CC=C1C2=CC(=O)C3=CC=CC=C3O2 | ||
| Standard InChIKey | YEHDMSUNJUONMW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O3/c1-18-14-8-4-3-7-12(14)16-10-13(17)11-6-2-5-9-15(11)19-16/h2-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

2'-Methoxyflavone Dilution Calculator

2'-Methoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9635 mL | 19.8177 mL | 39.6354 mL | 79.2707 mL | 99.0884 mL |
| 5 mM | 0.7927 mL | 3.9635 mL | 7.9271 mL | 15.8541 mL | 19.8177 mL |
| 10 mM | 0.3964 mL | 1.9818 mL | 3.9635 mL | 7.9271 mL | 9.9088 mL |
| 50 mM | 0.0793 mL | 0.3964 mL | 0.7927 mL | 1.5854 mL | 1.9818 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7927 mL | 0.9909 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- (S)-(-)-Limonene
Catalog No.:BCN9766
CAS No.:5989-54-8
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- Dodeca 2E,4E,8Z,10E,Z-tetraenoic acid isobutylamide
Catalog No.:BCN9763
CAS No.:866602-52-0
- 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one
Catalog No.:BCN9762
CAS No.:487-26-3
- Quinine sulfate dihydrate
Catalog No.:BCN9761
CAS No.:6119-70-6
- Rhoeadine
Catalog No.:BCN9760
CAS No.:2718-25-4
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- alpha-Hexylcinnamaldehyde
Catalog No.:BCN9779
CAS No.:101-86-0
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- Cannabisin B
Catalog No.:BCN9781
CAS No.:144506-17-2
Rare biscoumarin derivatives and flavonoids from Hypericum riparium.[Pubmed:24930002]
Phytochemistry. 2014 Sep;105:171-7.
Hypericum riparium A. Chev. is a Cameroonian medicinal plant belonging to the family Guttiferae. Chemical investigation of the methanol extract of the stem bark of H. riparium led to the isolation of four natural products, 7,7'-dihydroxy-6,6'-biscoumarin (1), 7,7'-dihydroxy-8,8'-biscoumarin (2), 7-methoxy-6,7'-dicoumarinyl ether (3), 2'-hydroxy-5'-(7''-methoxycoumarin-6''-yl)-4'-methoxyphenylpropanoic acid (4), together with one known 7,7'-dimethoxy-6,6'-biscoumarin (5), two flavones, 2'-methoxyflavone (6) and 3'-methoxy flavone (7), and two steroids, stigmast-4-en-3-one (8) and ergosta-4,6,8,22-tetraen-3-one (9). In addition, tetradecanoic acid (10), n-pentadecanoic acid (11), hexadecanoic acid (12), cis-10-heptadecenoic acid (13), octadecanoic acid (14) campesterol (15), stigmasterol (16), beta-sitosterol (17), stigmastanol (18), beta-eudesmol (19), 1-hexadecanol (20), and 1-octadecanol (21) were identified by GC-MS analysis. Compound 4 consists of a phenylpropanoic acid derivative fused with a coumarin unit, while compounds 2 and 3 are rare members of C8-C8' and C7-O-C6 linked biscoumarins. Their structures were elucidated by UV, IR, extensive 1D- and 2D-NMR experiments and electrospray (ESI) high resolution mass spectrometry (MS) including detailed MS/MS studies. This is the first report on the isolation of biscoumarins from the genus Hypericum, although simple coumarin derivatives have been reported from this genus in the literature. The cytotoxic activities of compounds 2-5 were evaluated against the human prostate cancer cell line PC-3 and the colon cancer cell line HT-29. They do not exhibit any significant cytotoxic activity.
Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition.[Pubmed:23432429]
Chem Res Toxicol. 2013 Apr 15;26(4):517-28.
A total of 68 chemicals including derivatives of naphthalene, phenanthrene, fluoranthene, pyrene, biphenyl, and flavone were examined for their abilities to interact with human P450s 2A13 and 2A6. Fifty-one of these 68 chemicals induced stronger Type I binding spectra (iron low- to high-spin state shift) with P450 2A13 than those seen with P450 2A6, i.e., the spectral binding intensities (DeltaAmax/Ks ratio) determined with these chemicals were always higher for P450 2A13. In addition, benzo[c]phenanthrene, fluoranthene, 2,3-dihydroxy-2,3-dihydrofluoranthene, pyrene, 1-hydroxypyrene, 1-nitropyrene, 1-acetylpyrene, 2-acetylpyrene, 2,5,2',5'-tetrachlorobiphenyl, 7-hydroxyflavone, chrysin, and galangin were found to induce a Type I spectral change only with P450 2A13. Coumarin 7-hydroxylation, catalyzed by P450 2A13, was strongly inhibited by 2'-methoxy-5,7-dihydroxyflavone, 2-ethynylnaphthalene, 2'-methoxyflavone, 2-naphththalene propargyl ether, acenaphthene, acenaphthylene, naphthalene, 1-acetylpyrene, flavanone, chrysin, 3-ethynylphenanthrene, flavone, and 7-hydroxyflavone; these chemicals induced Type I spectral changes with low Ks values. On the basis of the intensities of the spectral changes and inhibition of P450 2A13, we classified the 68 chemicals into eight groups based on the order of affinities for these chemicals and inhibition of P450 2A13. The metabolism of chemicals by P450 2A13 during the assays explained why some of the chemicals that bound well were poor inhibitors of P450 2A13. Finally, we compared the 68 chemicals for their abilities to induce Type I spectral changes of P450 2A13 with the Reverse Type I binding spectra observed with P450 1B1: 45 chemicals interacted with both P450s 2A13 and 1B1, indicating that the two enzymes have some similarty of structural features regarding these chemicals. Molecular docking analyses suggest similarities at the active sites of these P450 enzymes. These results indicate that P450 2A13, as well as Family 1 P450 enzymes, is able to catalyze many detoxication and activation reactions with chemicals of environmental interest.
Methoxyflavone derivatives modulate the effect of TRAIL-induced apoptosis in human leukemic cell lines.[Pubmed:22185222]
J Hematol Oncol. 2011 Dec 21;4:52.
BACKGROUND: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in various tumor cells, but does not affect normal cells or human leukemic cells, such as MOLT-4 and U937 cells, which are relatively resistant to TRAIL. Three flavonoids extracted from the rhizome of K. parviflora were 5,7-dimethoxyflavone (DMF), 5,7,4'-trimethoxyflavone (TMF) and 3,5,7,3',4'-pentamethoxyflavone (PMF), and synthetic flavonoids including 5-methoxyflavone (5-MF) and 2'-methoxyflavone (2"-MF) were chosen for testing in this study. The aims of this study were to examine whether the treatment of TRAIL-resistant leukemia MOLT-4 and U937 cells, with methoxyflavone derivatives could enhance the apoptotic response and to identify the mechanism involved. METHODS: The cytotoxic effect of methoxyflavone (MF) derivatives in MOLT-4, U937 and peripheral blood mononuclear cells (PBMCs) was analyzed by the MTT assay. The induction of apoptosis and the reduction of mitochondrial transmembrane potential (DeltaPsim) after staining with annexin V FITC and propidium iodide (PI), and 3,3'-dihexyloxacarbocyanine iodide (DiOC(6)), respectively, were performed using flow cytometry. ROS production was determined by staining with 2',7'-dichlorofluorescin diacetate and processed with a flow cytometer. DR4, DR5, cFLIP, Mcl-1, BAX and Bid expression were demonstrated by immunoblotting. Caspase-8 and -3 activities were determined by using IETD-AFC and DEVD-AFC substrates and the fluorescence intensity was measured. RESULTS: All methoxyflavone derivatives were cytotoxic to MOLT-4, U937 cells and PBMCs, except DMF, TMF and PMF were not toxic to PBMCs. All MF derivatives induced human leukemic MOLT-4 cell apoptosis, but not in U937 cells. Percentage of MOLT-4 cells with (DeltaPsim) was increased when treated with DMF, TMF, PMF, 5-MF and 2'-MF in the presence of TRAIL. 5-MF and 2'-MF enhanced TRAIL-induced apoptosis through the up-regulation of both DRs and the down-regulation of cFLIP and Mcl-1. Bid was cleaved and BAX was up-regulated, followed by the activation of caspase-8 and -3. Oxidative stress was also increased. 2'-MF gave the same result compared with 5-MF but with a less effect. CONCLUSION: Methoxyflavone derivatives enhanced TRAIL-induced apoptosis in human leukemic MOLT-4 cells through the death receptors and mitochondrial pathways.
Lipophilic flavones of Primula veris L. from field cultivation and in vitro cultures.[Pubmed:15896373]
Phytochemistry. 2005 May;66(9):1033-9.
Ten lipophilic flavones were isolated from the leaves of Primula veris from field cultivation - the newly described 3'-hydroxy-4',5'-dimethoxyflavone and 3'-methoxy-4',5'-methylenedioxyflavone, the previously known from chemical synthesis 3',4'-dimethoxyflavone, 2',5'-dimethoxyflavone, and also flavone, 2'-hydroxyflavone, 2'-methoxyflavone, 3'-methoxyflavone, 3',4',5'-trimethoxyflavone and 5,6,2',6'-tetramethoxyflavone (zapotin) which were previously known from plants. The same flavones were found in the leaves of P. veris obtained by in vitro propagation. The structural assignments were derived from (1)H NMR, (13)C NMR, EIMS and UV spectral data and the influence of B-ring oxygen substituents on the C-2, C-3 and H-3 NMR resonances in flavones unsubstituted in the A ring is taken into consideration.
Inhibition of [3H]-LSD binding to 5-HT7 receptors by flavonoids from Scutellaria lateriflora.[Pubmed:12713409]
J Nat Prod. 2003 Apr;66(4):535-7.
The hot water and 70% ethanol extracts of dried mad-dog skullcap (Scutellaria lateriflora) both bound to the 5-HT(7) receptor, with 87.2 +/- 6.2% and 56.7 +/- 1.3% inhibition of [(3)H]-LSD binding to the receptor at 100 microg/mL, respectively. The on-line analysis of a 70% ethanol extract by HPLC-UV/MS resulted in the identification of five flavones (1-5). Fractionation of the ethanol extract resulted in the isolation of three flavone-glucuronides (6-8) and a flavanone-glucuronide (9), including one new compound, lateriflorin (5,6,-dihydroxy-7-glucuronyloxy-2'-methoxyflavone) (8). The structure of 8 was determined by NMR ((1)H NMR, (13)C NMR, and NOESY experiments) and MS analysis. From the results obtained in the testing of the pure compounds, it is evident that the activity on the 5-HT(7) receptor is at least partly due to the presence of flavonoids. Scutellarin and ikonnikoside I showed the highest inhibition of [(3)H]-LSD binding with IC(50) values of 63.4 and 135.1 microM, respectively.
Studies on the constituents of Anaxagorea luzonensis A. GRAY.[Pubmed:10959592]
Chem Pharm Bull (Tokyo). 2000 Aug;48(8):1219-22.
Five new xanthones, 1,3,6-trihydroxy-5-methoxy-4-prenylxanthone (1), 1,3,5-trihydroxy-6-methoxy-2-prenylxanthone (2), 1,3,5-trihydroxy-4-(3-hydroxy-3-methylbutyl) xanthone (3), 1,3,6-trihydroxy-4-prenylxanthone (4), 3,6-dihydroxy-1,5-dimethoxyxanthone (5) and one new flavonoid, 3,5,7,4'-tetrahydroxy-2'-methoxyflavone (6) along with seven known xanthones and seven known flavonoids were isolated from the bark of Anaxagorea luzonensis A. GRAY and their chemical structures were determined by means of chemical and spectral studies. Almost all flavonoids and one xanthone (13) showed antioxidant activity.


