5-MethoxyflavanoneCAS# 55947-36-9 |
Quality Control & MSDS
Number of papers citing our products

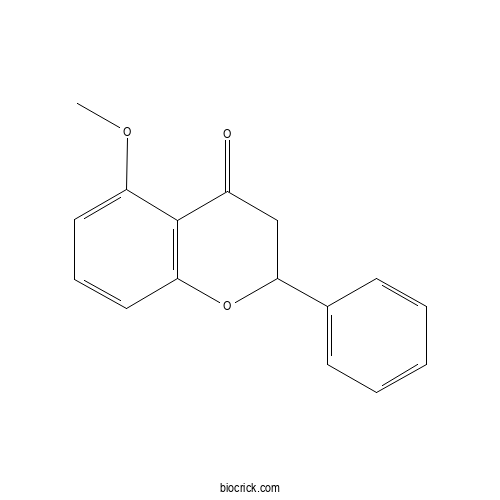
Chemical structure

3D structure
| Cas No. | 55947-36-9 | SDF | Download SDF |
| PubChem ID | 147795 | Appearance | Powder |
| Formula | C16H14O3 | M.Wt | 254.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-methoxy-2-phenyl-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC=CC2=C1C(=O)CC(O2)C3=CC=CC=C3 | ||
| Standard InChIKey | YLLFUILNISGLHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O3/c1-18-13-8-5-9-14-16(13)12(17)10-15(19-14)11-6-3-2-4-7-11/h2-9,15H,10H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-Methoxyflavone shows antioxidant and suppressive activity on the production of nitric oxide (NO) from lipopolysaccharide (LPS)-induced macrophage cells. It provides gastroprotection against nonsteroidal anti-inflammatory drug-induced gastric damage. 5-Methoxyflavanone induces cell cycle arrest at the G2/M phase, apoptosis and autophagy in HCT116 human colon cancer cells. | |||||

5-Methoxyflavanone Dilution Calculator

5-Methoxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9324 mL | 19.6618 mL | 39.3236 mL | 78.6473 mL | 98.3091 mL |
| 5 mM | 0.7865 mL | 3.9324 mL | 7.8647 mL | 15.7295 mL | 19.6618 mL |
| 10 mM | 0.3932 mL | 1.9662 mL | 3.9324 mL | 7.8647 mL | 9.8309 mL |
| 50 mM | 0.0786 mL | 0.3932 mL | 0.7865 mL | 1.5729 mL | 1.9662 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3932 mL | 0.7865 mL | 0.9831 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arganine B
Catalog No.:BCN9754
CAS No.:144425-21-8
- (+)-Menthofuran
Catalog No.:BCN9753
CAS No.:17957-94-7
- Benzyl acetate
Catalog No.:BCN9752
CAS No.:140-11-4
- 3',4',5,5',6,7-Hexamethoxyflavone
Catalog No.:BCN9751
CAS No.:29043-07-0
- Artemicapin C
Catalog No.:BCN9750
CAS No.:334007-19-1
- Neosalvianen
Catalog No.:BCN9749
CAS No.:790673-00-6
- Homoembelin
Catalog No.:BCN9748
CAS No.:38363-99-4
- 7-Methoxyflavonol
Catalog No.:BCN9747
CAS No.:7478-60-6
- 2-Naphthol
Catalog No.:BCN9746
CAS No.:135-19-3
- Hibifolin
Catalog No.:BCN9745
CAS No.:55366-56-8
- (+)-atechin 5-gallate
Catalog No.:BCN9744
CAS No.:128232-62-2
- Betonicine
Catalog No.:BCN9743
CAS No.:515-25-3
- 6,7-Dihydroxyflavone
Catalog No.:BCN9756
CAS No.:38183-04-9
- Octanoic acid
Catalog No.:BCN9757
CAS No.:124-07-2
- Ruberythric acid
Catalog No.:BCN9758
CAS No.:152-84-1
- Allylcysteine
Catalog No.:BCN9759
CAS No.:21593-77-1
- Rhoeadine
Catalog No.:BCN9760
CAS No.:2718-25-4
- Quinine sulfate dihydrate
Catalog No.:BCN9761
CAS No.:6119-70-6
- 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one
Catalog No.:BCN9762
CAS No.:487-26-3
- Dodeca 2E,4E,8Z,10E,Z-tetraenoic acid isobutylamide
Catalog No.:BCN9763
CAS No.:866602-52-0
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- (S)-(-)-Limonene
Catalog No.:BCN9766
CAS No.:5989-54-8
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
Cytotoxicity, Genotoxicity, Antioxidant Potential and Chemical Composition of Leaves of Campomanesia pubescens (Mart. ex DC.) O.Berg.[Pubmed:29943698]
Curr Pharm Biotechnol. 2018;19(5):416-421.
BACKGROUND: Plants of the genus Campomanesia belong to the family Myrtaceae and are very abundant in Cerrado areas. Teas from leaves of species of this genus are used for intestinal infections, combating obesity, stomach pathology, fever and among others. OBJECTIVE: The present study evaluated the chemical composition and antioxidant potential, cytotoxicity and genotoxicity of ethanolic extract from leaves of Campomanesia pubescens (Mart. ex DC.) O.Berg. METHOD: For the evaluation of antioxidant activity, the free radical DPPH and for determination of phenolic compounds Folin-Ciocalteau reagent were used. Identification of the substances was performed by HPLC-DAD by comparison of the retention times with standards analyzed under the same conditions and by evaluation of molecular absorption spectra in the ultraviolet and visible region. The cytotoxicity, genotoxicity were evaluated using Allium cepa bioassay. RESULTS: In the ethanolic extract 7-hydroxy-6-methyl-5-Methoxyflavanone, 5,7-dihydroxy-6-methylflavanone, 5,7-dihydroxy-8-methylflavanone, 2 ,4 -dihydroxy-6 -methoxychalcone, 5,7-dihydroxy-6,8- dimethylflavanone, 2 ,4 -dihydroxy-5 -methyl-6 -methoxychalcone and 2 ,4 -dihydroxy-3 ,5 -dimethyl-6 - methoxychalcone were identified. The extract showed antioxidant activity and cytotoxic effects on cell division and increased chromosomal alterations in Allium cepa test. CONCLUSION: These results showed antioxidant activity and suggest the cytotoxic and genotoxic effects in Allium cepa of ethanolic extract obtained from the leaves of Campomanesia pubescens.
Comparison of the Qualitative Chemical Composition of Extracts from Ageratina havanensis Collected in Two Different Phenological Stages by FIA-ESI-IT-MS" and UPLC/ESI-MS": Antiviral Activity.[Pubmed:30549819]
Nat Prod Commun. 2017 Jan;12(1):31-34.
The flowers and leaves of Ageratina havanensis (Kunth) R. M. King & H. Robinson are traditionally used as a tea to cure several diseases. The production of active secondary metabolites can be affected by several environmental factors such as climate, altitude, rainfall, phenological stage and other conditions that may influence the growth of plants. In this sense, the development of a methodology to compare the chemical composition of plant -extracts is needed. The qualitative chemical composition of the ethyl acetate extracts of flowers and leaves, collected in both reproductive and non-reproductive season, was determined by.flow injection analysis-electrospray ionization-ion trap tandem mass spectrometry (FIA-ESI-IT-MS") and ultra-high-performance liquid chromatography coupled to electrospray negative ionization mass spectrometry (UPLC/ESI-MS"). The qualitative chemical composition of the ethyl acetate extracts of flowers and leaves was very similar in all cases. Also the antiviral activity of flowers against human herpes simplex viruses type I and 2 (HSV-1, HSV-2) (Herpesviridae) was analyzed. Three glucoside flavonoids were isolated from the ethyl acetate extract of the leaves of A. havanensis collected in flowering season using chromatographic methods and their structures were elucidated by physical and spectroscopic data measurements, and by comparing the obtained data with previously published values. The compounds were identified as 3-Omicron-beta-D-glucosyl-7-methoxyaromadendrin (5), 7-Omicron-beta-D--glucosyl-4'- dihydroxy-5-Methoxyflavanone (6) and 5-O-beta-D-glucosylsakuranetin (7); this is the first report of the isolation of these compounds in the Asteraceae family. Since the qualitative composition of the extracts of A. havanensis was similar in all cases, it can be expected that the ethyl acetate extract of the leaves collected in the non-reproductive season has anti-herpetic activity similar to that obtained in the reproductive season.
[A novel C27-steroidal glycoside sulfate from Liriope graminifolia].[Pubmed:22812006]
Yao Xue Xue Bao. 2012 May;47(5):619-23.
An unusual novel C27-steroidal glycoside sulfate was isolated from the underground organs of Liriope graminifolia (Linn.) Baker with three known compounds. Their chemical structures were determined by spectral analysis, including HR-MS, 1D and 2D NMR as (25S)-ruscogenin 1-sulfate-3-O-alpha-L-rhamnopyranoside (1), (25S)-ruscogenin 1-O-beta-D-xylopyranosyl-3-O-alpha-L-rhamnopyranoside (2), hesperidin (3), and 4', 7-dihydroxy-5-Methoxyflavanone (4). Compound 1 has cytotoxic activities against K562 and HL60 cells with IC50 values of 18.6 microg x mL(-1) and 16.5 microg x mL(-1), respectively.
Isolation of novel phenolic compounds with multidrug resistance (MDR) reversal properties from Onychium japonicum.[Pubmed:21674783]
Chem Biodivers. 2011 Jun;8(6):1112-20.
We isolated seven novel compounds, namely, 3',4',6-trihydroxy-2,4-dimethoxy-3-(3'',4''-dihydroxybenzyl)chalcone (1), 3',6-dihydroxy-2,4,4'-trimethoxy-3-(3'',4''-dihydroxybenzyl)chalcone (2), alpha,beta-dihydro-3',6-dihydroxy-2,4,6'-trimethoxy-3-(3'',4''-dihydroxybenzyl)ch alcone (3), 3',4,4'-trihydroxy-2,6-dimethoxychalcone (4), 4',5,7-trihydroxy-6-(3'',4''-dihydroxybenzyl)flavone (5), 3-(3',4'-dihydroxybenzyl)-6,7-dihydroxycoumarin (6), 3-(3',4'-dihydroxyphenyl)-3,4-dihydroisocoumarin (7), as well as a known compound, 3',4',7-trihydroxy-5-Methoxyflavanone (8) from the whole grass of Onychium japonicum, and elucidated their structures by spectroscopic methods. Compounds 1-3 exhibited significant multidrug resistance (MDR) reversal effects on MCF-7/ADR and Bel-7402/5-Fu cell lines.
5-Methoxyflavanone induces cell cycle arrest at the G2/M phase, apoptosis and autophagy in HCT116 human colon cancer cells.[Pubmed:21616090]
Toxicol Appl Pharmacol. 2011 Aug 1;254(3):288-98.
Natural flavonoids have diverse pharmacological activities, including anti-oxidative, anti-inflammatory, and anti-cancer activities. In this study, we investigated the molecular mechanism underlying the action of 5-Methoxyflavanone (5-MF) which has a strong bioavailability and metabolic stability. Our results show that 5-MF inhibited the growth and clonogenicity of HCT116 human colon cancer cells, and that it activated DNA damage responses, as revealed by the accumulation of p53 and the phosphorylation of DNA damage-sensitive proteins, including ataxia-telangiectasia mutated (ATM) at Ser1981, checkpoint kinase 2 (Chk2) at Thr68, and histone H2AX at Ser139. 5-MF-induced DNA damage was confirmed in a comet tail assay. We also found that 5-MF increased the cleavage of caspase-2 and -7, leading to the induction of apoptosis. Pretreatment with the ATM inhibitor KU55933 enhanced 5-MF-induced gamma-H2AX formation and caspase-7 cleavage. HCT116 cells lacking p53 (p53(-/-)) or p21 (p21(-/-)) exhibited increased sensitivity to 5-MF compared to wild-type cells. 5-MF further induced autophagy via an ERK signaling pathway. Blockage of autophagy with the MEK inhibitor U0126 potentiated 5-MF-induced gamma-H2AX formation and caspase-2 activation. These results suggest that a caspase-2 cascade mediates 5-MF-induced anti-tumor activity, while an ATM/Chk2/p53/p21 checkpoint pathway and ERK-mediated autophagy act as a survival program to block caspase-2-mediated apoptosis induced by 5-MF.
Microbial metabolism. Part 10: Metabolites of 7,8-dimethoxyflavone and 5-methoxyflavone.[Pubmed:19731142]
Nat Prod Res. 2009;23(13):1231-9.
Microbial transformation of 7,8-dimethoxyflavone (1) by Mucor ramannianus produced five metabolites: 7,8-dimethoxy-4'-hydroxyflavone (2), 3',4'-dihydroxy-7,8-dimethoxyflavone (3), 7,3'-dihydroxy-8-methoxyflavone (4), 7,4'-dihydroxy-8-methoxyflavone (5) and 8-methoxy-7,3',4'-trihydroxyflavone (6). It was, however, completely converted to a single metabolite, 7-hydroxy-8-methoxyflavone (7) by Aspergillus flavus. 5-Methoxyflavone (8), when fermented with Beauveria bassiana, gave a single product, 5-Methoxyflavanone (9). Conversion of 8 with Aspergillus alliaceus yielded the metabolite 4'-hydroxy-5-methoxyflavone (10). The structures of the compounds 2-7, 9 and 10 were established by spectroscopic methods.
Studies on the constituents of Scutellaria species (XXII). Constituents of the roots of Scutellaria amabilis HARA.[Pubmed:16595941]
Chem Pharm Bull (Tokyo). 2006 Apr;54(4):435-41.
From the roots of Scutellaria amabilis HARA, eleven new flavonoids, 5,7,2'-trihydroxy-8-methoxyflavone 7-O-beta-D-glucopyranoside, 5,7,2'-trihydroxy-8-methoxyflavone 2'-O-beta-D-glucopyranoside, 5,7-dihydroxy-8,2'-dimethoxyflavone 7-O-beta-D-glucopyranoside, 5,7,2'-trihydroxyflavone 2'-O-beta-D-glucopyranoside, 5,7,2',5'-tetrahydroxyflavone 7-O-beta-D-glucuronopyranoside, (2S)-5,7,2',5'-tetrahydroxyflavanone, (2S)-5,7,2',5'-tetrahydroxyflavanone 7-O-beta-D-glucopyranoside, (2S)-5,7,2',5'-tetrahydroxyflavanone 7-O-beta-D-glucuronopyranoside, (2S)-7,2'-dihydroxy-5-Methoxyflavanone 7-O-beta-D-glucuronopyranoside, (I-2S)-I-5,II-5,I-7,II-7,I-2',II-2',II-5'-heptahydroxy-[I-6,II-6']-flavanonylflav one and (I-2S)-I-5,II-5,I-7,II-7,I-2',II-2',I-5',II-5'-octahydroxy-[I-6,II-6']-flavanonyl flavone, were isolated, together with ten known flavonoids, wogonin (5,7-dihydroxy-8-methoxyflavone), 5,7-dihydroxy-8,2'-dimethoxyflavone, (2S)-5,7,2'-trihydroxyflavanone, scutevulin (5,7,2'-trihydroxy-8-methoxyflavone), 5,7,4'-trihydroxy-8-methoxyflavone, alpinetin ((2S)-7-hydroxy-5-Methoxyflavanone), 5,7,2'-trihydroxyflavone, 5,7,2',5'-tetrahydroxyflavone, (2S)-7,2'-dihydroxy-5-Methoxyflavanone and 5,7-dihydroxy-8,2'-dimethoxyflavone 7-O-beta-D-glucuronopyranoside. The structures were determined on the basis of chemical and spectral data.
Effect of Chinese medicine alpinetin on the structure of human serum albumin.[Pubmed:15698801]
Bioorg Med Chem. 2005 Mar 1;13(5):1837-45.
Alpinetin (7-hydroxy-5-Methoxyflavanone), one of the main constituents from the seeds of Alpinia katsumadai Hayata, belongs to flavonoids with its usefulness as antibacterial, anti-inflammatory and other important therapeutic activities of significant potency and low systemic toxicity. In this paper, the interaction of alpinetin to human serum albumin (HSA) has been studied for the first time by spectroscopic method including Fourier transform infrared (FT-IR) spectroscopy, circular dichroism (CD), and UV-absorption spectroscopy in combination with fluorescence quenching study under physiological conditions with drug concentrations of 3.3 x 10(-6)-2.0 x 10(-5)mol/L. The results of spectroscopic measurements and the thermodynamic parameters obtained (the enthalpy change DeltaH(0) and the entropy change DeltaS(0) were calculated to be -10.20 kJ/mol and 53.97 J/molK(-1) according to the Van't Hoff equation) suggest that hydrophobic interaction is the predominant intermolecular forces stabilizing the complex, which is also good agreement with the results of molecule modeling study. The alterations of protein secondary structure in the presence of alpinetin in aqueous solution were quantitatively estimated by the evidences from FT-IR and CD spectroscopy with reductions of alpha-helices about 24%, decreases of beta-sheet structure about 2%, and increases of beta-turn structure about 21%. The quenching mechanism and the number of binding site (n approximately 1) were obtained by fluorescence titration data. Fluorescent displacement measurements confirmed that alpinetin bind HSA on site III. In addition, the effects of common ions on the constants of alpinetin-HSA complex were also discussed.
Identification and biological activity of microbial metabolites of xanthohumol.[Pubmed:14600365]
Chem Pharm Bull (Tokyo). 2003 Nov;51(11):1237-40.
Microbial transformation of xanthohumol using the culture broth of Cunninghamella echinulata NRRL 3655 afforded (2S)-8-[4"-hydroxy-3"-methyl-(2"-Z)-butenyl]-4',7-dihydroxy-5-Methoxyflavanone (5) and (2S)-8-[5"-hydroxy-3"-methyl-(2"-E)-butenyl]-4',7-dihydroxy-5-Methoxyflavanone (6). Xanthohumol (1) and flavanone 6 as well as (E)-2"-(2"'-hydroxyisopropyl)-dihydrofurano[2",3":4',3']-2',4-dihydroxy-6'-methox ychalcone (2), (2S)-2"-(2"'-hydroxyisopropyl)-dihydrofurano[2",3":7,8]-4'-hydroxy-5-methoxyflava none (3) obtained with Pichia membranifaciens showed antimalarial activity against Plasmodium falciparum.
Enantiomer separation of flavour and fragrance compounds by liquid chromatography using novel urea-covalent bonded methylated beta-cyclodextrins on silica.[Pubmed:12236512]
J Chromatogr A. 2002 Aug 30;968(1-2):31-40.
A novel methylated beta-cyclodextrin chiral stationary phase (CSP-ME), which was chemically immobilised onto porous silica via multiple urea-linkages was synthesised. The CSP-ME chiral stationary phase depicted good enantiomer separation abilities for some well-known flavour as well as fragrance compounds using high-performance liquid chromatography under reverse phase conditions. The optimum resolution for alpha-ionone, 3-methyl-alpha-ionone, flavanone, 5-Methoxyflavanone, 6-methoxyflavanone, 7-methoxyflavanone, hesperetin, naringenin and taxifolin was achieved using a mobile phase composition consisting of 1 wt.% triethylammonium acetate buffer (pH 4.68)-methanol. The effects of pH of triethylammonium acetate buffer and the methanol-acetonitrile content of the mobile phase composition on their retention time and resolution were examined to optimise the separation conditions.


