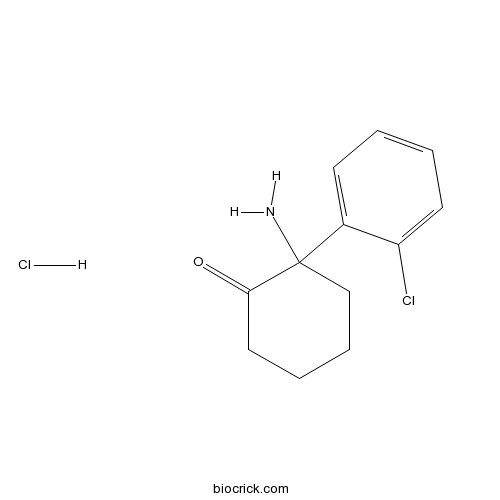
Norketamine hydrochlorideCAS# 79499-59-5 |
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- Pefloxacin
Catalog No.:BCC4231
CAS No.:70458-92-3
- Pefloxacin Mesylate
Catalog No.:BCC4821
CAS No.:70458-95-6
- Norfloxacin
Catalog No.:BCC4688
CAS No.:70458-96-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79499-59-5 | SDF | Download SDF |
| PubChem ID | 25195026 | Appearance | Powder |
| Formula | C12H15Cl2NO | M.Wt | 260.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 2-amino-2-(2-chlorophenyl)cyclohexan-1-one;hydrochloride | ||
| SMILES | C1CCC(C(=O)C1)(C2=CC=CC=C2Cl)N.Cl | ||
| Standard InChIKey | CLPOJGPBUGCUKT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H14ClNO.ClH/c13-10-6-2-1-5-9(10)12(14)8-4-3-7-11(12)15;/h1-2,5-6H,3-4,7-8,14H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Major metabolite of ketamine that is a potent non-competitive NMDA receptor antagonist (Ki = 3.6 μM for displacement of [3H]-MK 801 in rat brain). Antinociceptive and anesthetic in vivo. |

Norketamine hydrochloride Dilution Calculator

Norketamine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8438 mL | 19.2189 mL | 38.4379 mL | 76.8758 mL | 96.0947 mL |
| 5 mM | 0.7688 mL | 3.8438 mL | 7.6876 mL | 15.3752 mL | 19.2189 mL |
| 10 mM | 0.3844 mL | 1.9219 mL | 3.8438 mL | 7.6876 mL | 9.6095 mL |
| 50 mM | 0.0769 mL | 0.3844 mL | 0.7688 mL | 1.5375 mL | 1.9219 mL |
| 100 mM | 0.0384 mL | 0.1922 mL | 0.3844 mL | 0.7688 mL | 0.9609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glaucocalyxin A
Catalog No.:BCN2353
CAS No.:79498-31-0
- 9-Oxo-10,11-dehydroageraphorone
Catalog No.:BCN4333
CAS No.:79491-71-7
- Eleutheroside D
Catalog No.:BCN5336
CAS No.:79484-75-6
- Nicaraven
Catalog No.:BCC4684
CAS No.:79455-30-4
- Vernakalant
Catalog No.:BCC2036
CAS No.:794466-70-9
- PBP 10
Catalog No.:BCC6240
CAS No.:794466-43-6
- 360A
Catalog No.:BCC1307
CAS No.:794458-56-3
- Bruceantinoside A
Catalog No.:BCN7622
CAS No.:79439-85-3
- Yadanzioside P
Catalog No.:BCN6711
CAS No.:79439-84-2
- 3alpha-Cinnamoyloxypterokaurene L3
Catalog No.:BCN4575
CAS No.:79406-13-6
- 3alpha-Angeloyloxypterokaurene L3
Catalog No.:BCN4576
CAS No.:79406-11-4
- ent-3beta-Cinnamoyloxykaur-16-en-19-oic acid
Catalog No.:BCN1349
CAS No.:79406-10-3
- Stelleranol
Catalog No.:BCN8014
CAS No.:795308-62-2
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
- 5-Ethoxychelerthrine
Catalog No.:BCC8105
CAS No.:79559-55-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
- Crassicauline A
Catalog No.:BCN2516
CAS No.:79592-91-9
- Linifanib (ABT-869)
Catalog No.:BCC1261
CAS No.:796967-16-3
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
- H-Hyp(tBu)-OH
Catalog No.:BCC3249
CAS No.:79775-07-8
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
Oral ketamine is antinociceptive in the rat formalin test: role of the metabolite, norketamine.[Pubmed:10353496]
Pain. 1999 May;81(1-2):85-93.
The present study was designed to evaluate the oral efficacy and bioavailability of ketamine. Antinociceptive efficacy was determined with the rat formalin test and oral bioavailability by the measurement of plasma and brain concentrations of ketamine and its major metabolite, norketamine. Oral ketamine in a dose range from 30 to 180 mg/kg or saline was given prior to intraplantar formalin and the flinching behavior was measured. Oral ketamine dose-dependently reduced the flinching during phase 2, while flinching during phase 1 was reduced only with the highest dose given. Following oral ketamine at 100 mg/kg, blood and brain samples were obtained and plasma and brain ketamine and norketamine levels were measured using high-performance liquid chromatography (HPLC). The average concentration ratio of norketamine/ketamine, as expressed by the area under the curve (AUC) value, was 6.4 for plasma and 2.9 for brain. These results demonstrate that a significant amount of norketamine is formed by first pass biotransformation of ketamine and is distributed to the brain. Competition binding assays for the [3H]MK-801-labeled non-competitive site of the N-methyl-D-aspartate receptor (NMDA) receptor revealed that both norketamine and ketamine displaced [3H]MK-801 at low micromolar concentrations with Ki values of 2.5 and 0.3 mM in the forebrain, and 4.2 and 1.0 mM in the spinal cord, respectively. Spinal norketamine was approximately equipotent to ketamine in producing antinociceptive effects during phase 2 of the formalin test. Thus, norketamine appears to contribute to the antinociceptive effects of oral ketamine through its NMDA receptor antagonist activity.
Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord.[Pubmed:9311667]
Eur J Pharmacol. 1997 Aug 20;333(1):99-104.
The enantiomers of the potent non-competitive NMDA receptor antagonist ketamine and its major metabolite, norketamine were evaluated as NMDA receptor antagonists using the rat cortical wedge preparation and the neonatal rat spinal cord preparation, respectively, for electrophysiological studies and [3H](RS)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-im ine ([3H]MK801) in homogenate binding experiments. In agreement with earlier studies (S)-ketamine (Ki 0.3 microM) was found to possess a 5 times higher affinity for the NMDA receptor complex than (R)-ketamine (Ki 1.4 microM). (S)-Norketamine (Ki 1.7 microM) had approximately an 8 times higher affinity than (R)-norketamine (Ki 13 microM) in the inhibition of [3H]MK-801 binding. All compounds inhibited responses to NMDA in the rat cortical wedge preparation and the hemisected neonatal rat spinal cord, being approximately four times more potent in the cortex than in the spinal cord except for (R)-norketamine being only twice as potent. In light of the clinically obtained concentrations of norketamine after oral administration of ketamine, these data strongly suggest that (S)-norketamine may contribute significantly to the clinical activity of (S)-ketamine, especially when given orally.
Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine.[Pubmed:3783598]
J Med Chem. 1986 Nov;29(11):2396-9.
(Z)-6-Hydroxynorketamine (3), a secondary metabolite of the dissociative anesthetic agent ketamine (1), was synthesized, and its central nervous system (CNS) properties were compared to those of the parent drug and the primary metabolite, norketamine (2). Administration of compounds 1 and 2 to rats (40 mg/kg iv) produced general anesthesia and also led to marked increases in spontaneous locomotor activity during the postanesthetic recovery phase. These effects were of significantly longer duration with 1 than with 2. In contrast, the same dose of 3 produced neither general anesthesia nor CNS excitation, despite the fact that 3 entered brain tissue readily from the systemic circulation. It is concluded that the CNS effects of 1 are attenuated by metabolism to 2 and are abolished by subsequent hydroxylation to produce 3. Moreover, the results suggest that the desirable anesthetic properties of 1 and related arylcyclohexylamines may be inseparable from their ability to produce adverse postanesthetic emergence reactions.


