9-Oxo-10,11-dehydroageraphoroneCAS# 79491-71-7 |
Quality Control & MSDS
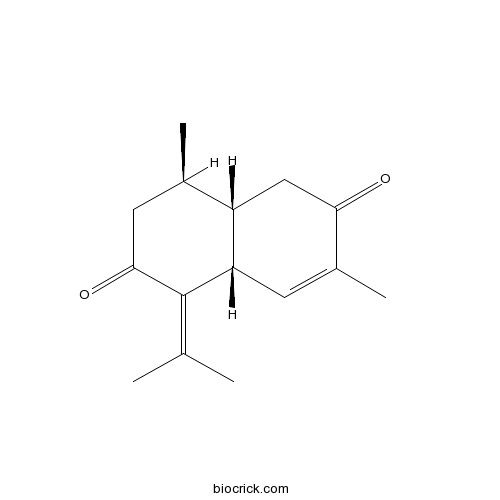
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79491-71-7 | SDF | Download SDF |
| PubChem ID | 91885218 | Appearance | Powder |
| Formula | C15H20O2 | M.Wt | 232.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aR,8R,8aS)-3,8-dimethyl-5-propan-2-ylidene-4a,7,8,8a-tetrahydro-1H-naphthalene-2,6-dione | ||
| SMILES | CC1CC(=O)C(=C(C)C)C2C1CC(=O)C(=C2)C | ||
| Standard InChIKey | RDQAKTSDUZUBQC-USWWRNFRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 9-Oxo-10,11-dehydroageraphorone has acaricidal activity. 2. 9-Oxo-10,11-dehydroageraphorone induces hepatotoxicity and cholestasis in rats. 3. 9-Oxo-10,11-dehydroageraphorone effectively inhibits the proliferation of HeLa cells by arresting the cell cycle transition from S to G2/M phase. |
| Targets | Caspase | Antifection |

9-Oxo-10,11-dehydroageraphorone Dilution Calculator

9-Oxo-10,11-dehydroageraphorone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3048 mL | 21.5239 mL | 43.0478 mL | 86.0956 mL | 107.6195 mL |
| 5 mM | 0.861 mL | 4.3048 mL | 8.6096 mL | 17.2191 mL | 21.5239 mL |
| 10 mM | 0.4305 mL | 2.1524 mL | 4.3048 mL | 8.6096 mL | 10.7619 mL |
| 50 mM | 0.0861 mL | 0.4305 mL | 0.861 mL | 1.7219 mL | 2.1524 mL |
| 100 mM | 0.043 mL | 0.2152 mL | 0.4305 mL | 0.861 mL | 1.0762 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eleutheroside D
Catalog No.:BCN5336
CAS No.:79484-75-6
- Nicaraven
Catalog No.:BCC4684
CAS No.:79455-30-4
- Vernakalant
Catalog No.:BCC2036
CAS No.:794466-70-9
- PBP 10
Catalog No.:BCC6240
CAS No.:794466-43-6
- 360A
Catalog No.:BCC1307
CAS No.:794458-56-3
- Bruceantinoside A
Catalog No.:BCN7622
CAS No.:79439-85-3
- Yadanzioside P
Catalog No.:BCN6711
CAS No.:79439-84-2
- 3alpha-Cinnamoyloxypterokaurene L3
Catalog No.:BCN4575
CAS No.:79406-13-6
- 3alpha-Angeloyloxypterokaurene L3
Catalog No.:BCN4576
CAS No.:79406-11-4
- ent-3beta-Cinnamoyloxykaur-16-en-19-oic acid
Catalog No.:BCN1349
CAS No.:79406-10-3
- ent-3Beta-Tigloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1350
CAS No.:79406-09-0
- BIBU 1361 dihydrochloride
Catalog No.:BCC7356
CAS No.:793726-84-8
- Glaucocalyxin A
Catalog No.:BCN2353
CAS No.:79498-31-0
- Norketamine hydrochloride
Catalog No.:BCC5859
CAS No.:79499-59-5
- Stelleranol
Catalog No.:BCN8014
CAS No.:795308-62-2
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
- 5-Ethoxychelerthrine
Catalog No.:BCC8105
CAS No.:79559-55-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
- Alarelin Acetate
Catalog No.:BCC1336
CAS No.:79561-22-1
- Crassicauline A
Catalog No.:BCN2516
CAS No.:79592-91-9
- Linifanib (ABT-869)
Catalog No.:BCC1261
CAS No.:796967-16-3
- Levonorgestrel
Catalog No.:BCC4792
CAS No.:797-63-7
Induction and mechanism of HeLa cell apoptosis by 9-oxo10, 11-dehydroageraphorone from Eupatorium adenophorum.[Pubmed:25647450]
Oncol Rep. 2015 Apr;33(4):1823-7.
9-Oxo-10, 11-dehydroageraphorone (euptox A), a cadenine sesquiterpene, is the main toxin from Eupatorium adenophorum. The aim of the present study was to examine the induction and mechanism of HeLa cell apoptosis by euptox A. The apoptosisinducing effect of the euptox A on HeLa cells was examined by MTT assay. The underlying mechanism was analyzed by flow cytometry and quantitative PCR. Flow cytometry results suggested that euptox A effectively inhibited the proliferation of HeLa cells, arrested the cell cycle transition from S to G2/M phase, did not continue to complete the cell cycle activity (mainly from 4 times and mitosis), and induced cell proliferation. The RT-qPCR detection results showed that euptox A induced apoptosis by improving the gene expression level of apoptotic proteases such as caspase-10 in HeLa cells. Its mechanism of action was associated with the upregulation of apoptotic gene expression and arresting of the cell cycle.
Clinical efficacy of 9-oxo-10, 11-dehydroageraphorone extracted from Eupatorium adenophorum against Psoroptes cuniculi in rabbits.[Pubmed:25527276]
BMC Vet Res. 2014 Dec 20;10:970.
BACKGROUND: Animal acariasis is one of the important veterinary skin diseases. Chemical drugs have been widely used to treat and control this kind of disease. But many chemicals control could increase resistance in target species, toxicity and environmental hazards. We found that the 9-oxo-10, 11-dehydroageraphorone (euptox A) extracted from E. adenophorum has strong toxicity against P. cuniculi in vitro, but the in vivo acaricidal actions of euptox A have yet to be investigated. RESULTS: A 14-day experiment was performed using rabbits that were naturally infested with P. cuniculi on a farm. Rabbits were randomly divided into five groups; animals in groups A, B and C were treated in each ear topically with 4.0 ml of 2.0 and 1.0 g/L (w/v) euptox A, respectively. Animals in groups D and E were treated with ivermectin (by injection; positive controls) and glycerol with water only (by embrocation; negative controls), respectively. Each rabbit was treated twice with separate treatments on days 0 and 7. Rabbits were observed daily and detailed examinations were performed on days 0, 7 and 14, to inspect the presence or absence of mites and scabs/crusts. Seven days after the initial treatment, the mean clinical scores (presence of scabs/crusts) decreased from 3.48, 3.37, 3.43 and 3.45 to 0.37, 0.42, 0.78 and 0.38 in the ears of animals in groups A, B , C and D, respectively, which were similar to the observations recorded in the positive control rabbits. However, the clinical score for negative control rabbits did not increase significantly (P > 0.05) during the experiment, and this changed from 3.32 to 3.37 in the ears, and there were no significant differences in clinical efficacy between left and right ears. After two treatments (0 and 7 d), the rabbits in groups A, B, C and D had recovered completely 14 days after the last treatment and no recurrences of infection were observed. CONCLUSIONS: These results indicate that euptox A was potent compounds for the effective control of animal P. cuniculi in vivo.
Hepatotoxicity and cholestasis in rats induced by the sesquiterpene, 9-oxo-10,11-dehydroageraphorone, isolated from Eupatorium adenophorum.[Pubmed:11835625]
J Biochem Mol Toxicol. 2001;15(5):279-86.
Eupatorium adenophorum leaves cause hepatotoxicity and cholestasis in rats. The hepatotoxicant has been characterized as 9-Oxo-10,11-dehydroageraphorone (ODA), a cadinene sesquiterpene. Oral administration of ODA, mixed in feed to rats, caused jaundice in 24 h. The liver of the intoxicated animals had focal areas of hepatocellular necrosis, proliferation, and dilation of bile ducts with degenerative changes in the lining epithelium. There was marked increase in the conjugated form of plasma bilirubin and in the activities of the enzymes glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, alkaline phosphatase, lactate dehydrogenase, gamma-glutamyltranspeptidase, glutamate dehydrogenase, and 5'-nucleotidase. The histopathological lesions in liver and biochemical profile of marker enzymes show that ODA induced hepatotoxicity and cholestasis in rats. This is the first report on the toxicity of a cadinene sesquiterpene in rats.
Acaricidal activity of 9-oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum in vitro.[Pubmed:24631419]
Exp Parasitol. 2014 May;140:8-11.
The acaricidal activity of the 9-Oxo-10,11-dehydroageraphorone (euptox A), a cadenine sesquiterpene from Eupatorium adenophorum (E. adenophorum) against Sarcoptes scabiei and Psoroptes cuniculi was tested in vitro. A complementary log-log (CLL) model was used to analyze the data of the toxicity tests in vitro. The results showed euptox A had strong toxicity against mites, killing all S. scabiei at 3 and 4 mg/ml (m/v) concentration, while 4 mg/ml euptox A was also found to kill all P. cuniculi within a 4 h period. Similarly, 2, 3 and 4 mg/ml concentration of euptox A had strong toxicity against S. scabiei, with median lethal time (LT50) values at 0.687, 0.526, 0.326 h, respectively. 3 mg/ml and 4 mg/ml showed strong acaricidal action against P. cuniculi; the LT50 values were 0.693 and 0.493 h, respectively. The median lethal concentration (LC50) values were 1.068 mg/ml for Scabies mite and 0.902 mg/ml for P. cuniculi in 2 h. The results indicate that euptox A has strong acaricidal activity and may exploit as novel drugs for the effective control of animal acariasis.


