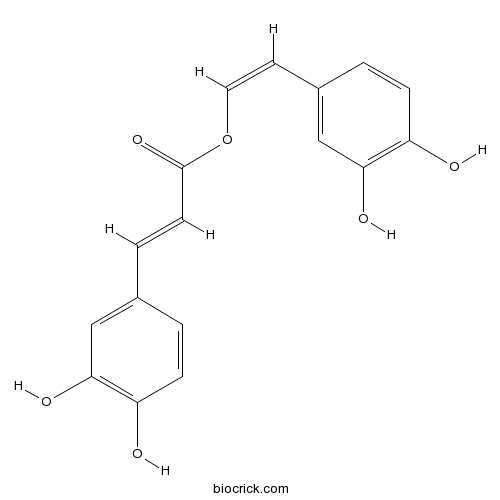
Nepetoidin BCAS# 55486-06-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55486-06-1 | SDF | Download SDF |
| PubChem ID | 5316819 | Appearance | Powder |
| Formula | C17H14O6 | M.Wt | 314.29 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(Z)-2-(3,4-dihydroxyphenyl)ethenyl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)OC=CC2=CC(=C(C=C2)O)O)O)O | ||
| Standard InChIKey | GFZFUVWXGQWUGX-DGEKEWMVSA-N | ||
| Standard InChI | InChI=1S/C17H14O6/c18-13-4-1-11(9-15(13)20)3-6-17(22)23-8-7-12-2-5-14(19)16(21)10-12/h1-10,18-21H/b6-3+,8-7- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Nepetoidin B has anti-fungal and anti-bacterial effects. 2. Nepetoidin B shows antiinflammatory effect, it can inhibit LPS-stimulated NO production possibly via modulation of iNOS mediated by MKP-5/NF-κB pathways in RAW 264.7 cells. |
| Targets | NO | NOS | TNF-α | IL Receptor | NF-kB | p65 | COX | JNK | p38MAPK | Antifection |

Nepetoidin B Dilution Calculator

Nepetoidin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1818 mL | 15.9089 mL | 31.8177 mL | 63.6355 mL | 79.5444 mL |
| 5 mM | 0.6364 mL | 3.1818 mL | 6.3635 mL | 12.7271 mL | 15.9089 mL |
| 10 mM | 0.3182 mL | 1.5909 mL | 3.1818 mL | 6.3635 mL | 7.9544 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6364 mL | 1.2727 mL | 1.5909 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6364 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mollugin
Catalog No.:BCN5742
CAS No.:55481-88-4
- Isosaxalin
Catalog No.:BCN5741
CAS No.:55481-86-2
- Indole-3-glyoxylamide
Catalog No.:BCN6802
CAS No.:5548-10-7
- Jujuboside B
Catalog No.:BCN4950
CAS No.:55466-05-2
- Jujuboside A
Catalog No.:BCN4949
CAS No.:55466-04-1
- Chimonanthine
Catalog No.:BCN7824
CAS No.:5545-89-1
- Z-Asp(OtBu)-OH.H2O
Catalog No.:BCC2789
CAS No.:5545-52-8
- Anisodamine hydrobromide
Catalog No.:BCC8119
CAS No.:55449-49-5
- Boc- D-1-Nal-OH
Catalog No.:BCC3283
CAS No.:55447-00-2
- 8beta-(4-Hydroxytigloyloxy)ovatifolin
Catalog No.:BCN7122
CAS No.:554449-27-3
- Deferasirox Fe3+ chelate
Catalog No.:BCC1521
CAS No.:554435-83-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- Myriceric acid B
Catalog No.:BCN5743
CAS No.:55497-79-5
- Methyldopa
Catalog No.:BCC4676
CAS No.:555-30-6
- Tritetradecanoin
Catalog No.:BCN8389
CAS No.:555-45-3
- CCCP
Catalog No.:BCC5658
CAS No.:555-60-2
- 6-Shogaol
Catalog No.:BCN6288
CAS No.:555-66-8
- Biondinin C
Catalog No.:BCN5744
CAS No.:55511-08-5
- H-1-Nal-OH
Catalog No.:BCC3282
CAS No.:55516-54-6
- Boc-Phe(4-NH2)-OH
Catalog No.:BCC3153
CAS No.:55533-24-9
- Agrimol B
Catalog No.:BCN5017
CAS No.:55576-66-4
- Acesulfame Potassium
Catalog No.:BCC4755
CAS No.:55589-62-3
- H-D-Tyr-OH
Catalog No.:BCC3134
CAS No.:556-02-5
- Alliin
Catalog No.:BCN3869
CAS No.:556-27-4
The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae.[Pubmed:12943769]
Phytochemistry. 2003 Sep;64(2):519-28.
A survey of leaf surface constituents in the family Lamiaceae using HPLC with diode array detection revealed the presence of two characteristic phenolic compounds in many species. The distribution of these phenolics in the Lamiaceae was found to be of taxonomic significance, as they were present in the great majority of species investigated for the subfamily Nepetoideae, including representatives of the well-known genera of culinary herbs, mint, rosemary, sage, thyme and basil. In contrast, they were absent from species of the other subfamilies of Lamiaceae studied and from the related families Verbenaceae, Scrophulariaceae, Acanthaceae and Buddlejaceae. The compounds were isolated from Plectranthus crassus and identified by NMR spectroscopy as the known caffeic acid esters (Z,E)-[2-(3,5-dihydroxyphenyl)ethenyl] 3-(3,4-dihydroxyphenyl)-2-propenoate and (Z,E)-[2-(3,4-dihydroxyphenyl)ethenyl] 3-(3,4-dihydroxyphenyl)-2-propenoate, for which the trivial names nepetoidins A and B are proposed. The presence of this pair of caffeic acid esters adds another character to the chemical, palynological and embryological features distinguishing the Nepetoideae from the other subfamilies of Lamiaceae and related families, and supports the view that the Nepetoideae are a specialised and monophyletic group within the family. Nepetoidin B was shown to have a greater antioxidant activity than gallic, rosmarinic and caffeic acids, and showed activity as an insect phagostimulant. Both compounds were antifungal.
Nepetoidin B, a Natural Product, Inhibits LPS-stimulated Nitric Oxide Production via Modulation of iNOS Mediated by NF-kappaB/MKP-5 Pathways.[Pubmed:28504466]
Phytother Res. 2017 Jul;31(7):1072-1077.
Previous reports showed that Nepetoidin B (NTB), a natural product isolated from many herbs, has anti-fungal and anti-bacterial effects. In this study, the antiinflammatory effect of NTB was investigated in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. The cytotoxic effect of NTB and LPS was determined by MTT assay. The nitric oxide (NO) production was detected by Griess assay. The TNF-alpha and IL-6 levels were determined by enzyme-linked immunosorbent assay kits. Protein expressions were tested by western blotting. The transcription activity of inducible nitric oxide synthase (iNOS) was detected by luciferase assay. Immunofluorescence assay was used to observe the visualization of NF-kappaB/p65 nuclear translocation. NTB and LPS showed no obvious cytotoxic effect on RAW 264.7 cells. NTB remarkably inhibited LPS-induced NO and TNF-alpha secretion in a concentration-dependent manner while showed no significant effect on IL-6 secretion. NTB inhibited LPS-induced iNOS protein expression and transcription activity without affecting cyclooxygenase-2. Furthermore, NTB suppressed LPS-stimulated NF-kappaB/p65 phosphorylation and nuclear translocation. In addition, NTB significantly inhibited LPS-induced phosphorylation of JNK1/2 and p38MAPK without affecting ERK1/2. LPS-induced inhibition of mitogen-activated protein kinase phosphatase-5 (MKP-5) was completely reversed by NTB. In conclusion, these results suggested that NTB inhibited LPS-stimulated NO production possibly via modulation of iNOS mediated by MKP-5/NF-kappaB pathways in RAW 264.7 cells. Copyright (c) 2017 John Wiley & Sons, Ltd.
Antileishmanial Phenylpropanoids from the Leaves of Hyptis pectinata (L.) Poit.[Pubmed:23983783]
Evid Based Complement Alternat Med. 2013;2013:460613.
Hyptis pectinata, popularly known in Brazil as "sambacaita" or "canudinho," is an aromatic shrub largely grown in the northeast of Brazil. The leaves and bark are used in an infusion for the treatment of throat and skin inflammations, bacterial infections, pain, and cancer. Analogues of rosmarinic acid and flavonoids were obtained from the leaves of Hyptis pectinata and consisted of two new compounds, sambacaitaric acid (1) and 3-O-methyl-sambacaitaric acid (2), and nine known compounds, rosmarinic acid (3), 3-O-methyl-rosmarinic acid (4), ethyl caffeate (5), nepetoidin A (6), Nepetoidin B (7), cirsiliol (8), circimaritin (9), 7-O-methylluteolin (10), and genkwanin (11). The structures of these compounds were determined by spectroscopic methods. Compounds 1-5, and 7 were evaluated in vitro against the promastigote form of L. braziliensis, and the ethanol extract. The hexane, ethyl acetate, and methanol-water fractions were also evaluated. The EtOH extract, the hexane extract, EtOAc, MeOH:H2O fractions; and compounds 1, 2 and 4 exhibited antileishmanial activity, and compound 1 was as potent as pentamidine. In contrast, compounds 3, 5, and 7 did not present activity against the promastigote form of L. braziliensis below 100 microM. To our knowledge, compounds 1 and 2 are being described for the first time.


