CCCPuncoupler of oxidative phosphorylation CAS# 555-60-2 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 555-60-2 | SDF | Download SDF |
| PubChem ID | 2603 | Appearance | Powder |
| Formula | C9H5ClN4 | M.Wt | 204.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Carbonyl cyanide 3-chlorophenylhydrazone; Carbonyl Cyanide m-Chlorophenylhydrazone | ||
| Solubility | DMSO : ≥ 100 mg/mL (488.71 mM) *"≥" means soluble, but saturation unknown. | ||
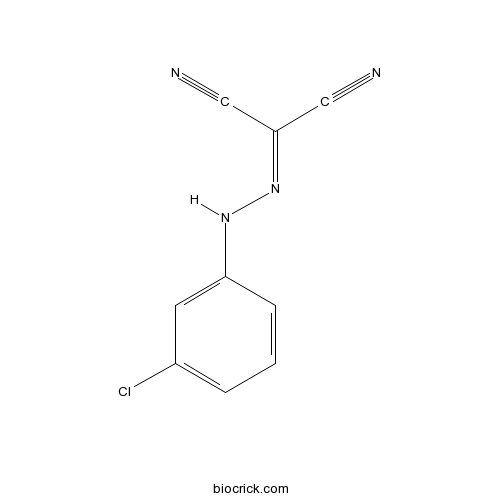
| Chemical Name | 2-[(3-chlorophenyl)hydrazinylidene]propanedinitrile | ||
| SMILES | C1=CC(=CC(=C1)Cl)NN=C(C#N)C#N | ||
| Standard InChIKey | UGTJLJZQQFGTJD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H5ClN4/c10-7-2-1-3-8(4-7)13-14-9(5-11)6-12/h1-4,13H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Widely used uncoupler of oxidative phosphorylation. |

CCCP Dilution Calculator

CCCP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8871 mL | 24.4355 mL | 48.8711 mL | 97.7422 mL | 122.1777 mL |
| 5 mM | 0.9774 mL | 4.8871 mL | 9.7742 mL | 19.5484 mL | 24.4355 mL |
| 10 mM | 0.4887 mL | 2.4436 mL | 4.8871 mL | 9.7742 mL | 12.2178 mL |
| 50 mM | 0.0977 mL | 0.4887 mL | 0.9774 mL | 1.9548 mL | 2.4436 mL |
| 100 mM | 0.0489 mL | 0.2444 mL | 0.4887 mL | 0.9774 mL | 1.2218 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
CCCP (carbonyl cyanide m-chlorophenyl hydrazine) is widely used uncoupler of oxidative phosphorylation.
The uncoupler of oxidative phosphorylation blocks ATP synthesis by collapsing the proton motive force.
In vitro: A genetic β-galactoside reporter system with a disk diffusion assay on MacConkey Lactose agar petri plates to monitor maintenance of the bacteriophage λ prophage state and viral induction in Escherichia coli K-12. It presented evidence that the phage λ major lytic promoters, pL and pR, are activated via cells containing the reporters following exposure to the energy poison CCCP. Requirement of expression of the λ lytic promoters in response to CCCP is host RecA function and an auto-cleavable CI repressor, as does SOS induction of the λ prophage occurring by a DNA damage-dependent pathway. CCCP-mediated activation of the λ lytic promoters required λ Cro function. CCCP does not modulate an sfi-lacZ SOS reporter [1]. The uncoupler CCCP blocks oxidative phosphorylation by damaging the proton gradient. An anion CCCP can bind a proton. but CCCP with a delocalized negative charge allows it to cross the lipid bilayer in the unprotonated form, while weak acids cross the hydrophobic membrane only when protonated. Many protons can be transported by one molecule of CCCP across the inner membrane. It is described that a genetic reporter system to monitor maintenance of the λ prophage state and viral induction, and evidence is presented that it activates the major leftward and rightward lytic promoters of the λ prophage that cells are exposed to the energy poison CCCP.
In vivo: So far, no study in vivo has been conducted.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1]. Thomason LC, Court DL. Evidence that bacteriophage λ lysogens may induce in response to the proton motive force uncoupler CCCP. FEMS Microbiol Lett. 2016 Feb;363(3).
- Tritetradecanoin
Catalog No.:BCN8389
CAS No.:555-45-3
- Methyldopa
Catalog No.:BCC4676
CAS No.:555-30-6
- Myriceric acid B
Catalog No.:BCN5743
CAS No.:55497-79-5
- Nepetoidin B
Catalog No.:BCN7082
CAS No.:55486-06-1
- Mollugin
Catalog No.:BCN5742
CAS No.:55481-88-4
- Isosaxalin
Catalog No.:BCN5741
CAS No.:55481-86-2
- Indole-3-glyoxylamide
Catalog No.:BCN6802
CAS No.:5548-10-7
- Jujuboside B
Catalog No.:BCN4950
CAS No.:55466-05-2
- Jujuboside A
Catalog No.:BCN4949
CAS No.:55466-04-1
- Chimonanthine
Catalog No.:BCN7824
CAS No.:5545-89-1
- Z-Asp(OtBu)-OH.H2O
Catalog No.:BCC2789
CAS No.:5545-52-8
- Anisodamine hydrobromide
Catalog No.:BCC8119
CAS No.:55449-49-5
- 6-Shogaol
Catalog No.:BCN6288
CAS No.:555-66-8
- Biondinin C
Catalog No.:BCN5744
CAS No.:55511-08-5
- H-1-Nal-OH
Catalog No.:BCC3282
CAS No.:55516-54-6
- Boc-Phe(4-NH2)-OH
Catalog No.:BCC3153
CAS No.:55533-24-9
- Agrimol B
Catalog No.:BCN5017
CAS No.:55576-66-4
- Acesulfame Potassium
Catalog No.:BCC4755
CAS No.:55589-62-3
- H-D-Tyr-OH
Catalog No.:BCC3134
CAS No.:556-02-5
- Alliin
Catalog No.:BCN3869
CAS No.:556-27-4
- Alverine Citrate
Catalog No.:BCC4619
CAS No.:5560-59-8
- 1-Oxo-4-hydroxy-2-en-4-ethylcyclohexa-5,8-olide
Catalog No.:BCN1417
CAS No.:55604-88-1
- Aristololactam II
Catalog No.:BCN8095
CAS No.:55610-00-9
- Cepharadione A
Catalog No.:BCN3950
CAS No.:55610-01-0
p62 prevents carbonyl cyanide m-chlorophenyl hydrazine (CCCP)-induced apoptotic cell death by activating Nrf2.[Pubmed:26208452]
Biochem Biophys Res Commun. 2015 Sep 4;464(4):1139-1144.
Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) is a mitochondrial depolarizing agent that induces reactive oxygen species (ROS)-mediated cell death. The Nrf2-Keap1 pathway is crucial for the elimination of ROS in stressed cells. However, the molecular mechanism underlying the regulation of the Nrf2-Keap1 pathway in CCCP-induced cell death is unknown. In this study, we demonstrated that CCCP promotes Keap1 degradation, and thereby activates Nrf2. This CCCP-mediated Keap1 degradation is partly dependent on autophagy. Moreover, CCCP-induced Keap1 degradation is mainly reliant on p62, which functions as an adaptor protein during selective autophagy. Lack of p62 blocked CCCP-induced Keap1 degradation and inhibited Nrf2 activation, and thereby increased the accumulation of ROS. Ablation of p62 increased the susceptibility of cells to oxidative stress. These results indicate that p62 plays an important role in protecting cells against oxidative stress through Keap1 degradation-mediated Nrf2 activation.
Melanoma differentiation-associated gene 5 is involved in the induction of stress granules and autophagy by protonophore CCCP.[Pubmed:26351918]
Biol Chem. 2016 Jan;397(1):67-74.
The eukaryotic cell has evolved a variety of stress responses against external stimuli, such as innate immunity, the formation of stress granules (SGs), and autophagy. We previously demonstrated that the innate immune adaptor IFN-beta promoter stimulator 1 (IPS-1) plays an essential role in the formation of dsRNA-induced SGs, indicating a connection between SG formation and innate immunity. In this study, it was further demonstrated that melanoma differentiation-associated gene 5 (MDA5), an innate immune sensor, is involved in SG formation induced by carbonyl cyanide m-chlorophenylhydrazone (CCCP), a mitochondrial protonophore. MDA5 knockdown had no significant impact on the phosphorylation of eukaryotic translation initiation factor 2alpha (eIF2alpha) triggered by CCCP, and MDA5 itself was not recruited to SGs, suggesting that the regulation of MDA5 in the SG response occurs downstream of eIF2alpha. Furthermore, the depletion of MDA5 or G3BP1 led to reduced autophagy in CCCP-stimulated cells, implying that the regulatory effect of MDA5 with respect to autophagy depends on its role in SG formation. This study uncovered an unexpected role of the innate immune protein MDA5 in SG formation and autophagy triggered by the protonophore CCCP, further supporting a correlation between different stress responses.
Evidence that bacteriophage lambda lysogens may induce in response to the proton motive force uncoupler CCCP.[Pubmed:26705574]
FEMS Microbiol Lett. 2016 Feb;363(3). pii: fnv244.
We describe a genetic beta-galactoside reporter system using a disk diffusion assay on MacConkey Lactose agar petri plates to monitor maintenance of the bacteriophage lambda prophage state and viral induction in Escherichia coli K-12. Evidence is presented that the phage lambda major lytic promoters, pL and pR, are activated when cells containing the reporters are exposed to the energy poison carbonyl cyanide m-chlorophenyl hydrazine, CCCP. This uncoupler of oxidative phosphorylation inhibits ATP synthesis by collapsing the proton motive force. Expression of the lambda lytic promoters in response to CCCP requires host RecA function and an autocleavable CI repressor, as does SOS induction of the lambda prophage that occurs by a DNA damage-dependent pathway. lambda Cro function is required for CCCP-mediated activation of the lambda lytic promoters. CCCP does not induce an sfi-lacZ SOS reporter.
Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) Reverses Resistance to Colistin, but Not to Carbapenems and Tigecycline in Multidrug-Resistant Enterobacteriaceae.[Pubmed:28261184]
Front Microbiol. 2017 Feb 14;8:228.
Background: Carbapenems (CAR), colistin (CST), and tigecycline (TGC) alone or in combination therapy has become the last-resort antibiotics for treating infections caused by multidrug resistant (MDR) bacteria. However, resistance to these reserve antibiotics are increasingly being reported worldwide. Hence, the quest to find other agents that will synergistically restore the efficacy of these antibiotics have increased. Methods: Sixty-three clinical Enterobacteriaceae isolates comprising of Klebsiella pneumoniae (n = 24), Enterobacter spp. (n = 15), Serratia marcescens (n = 12), Citrobacter freundii (n = 8), Escherichia coli (n = 2), and K. oxytoca/michiganensis (n = 2) with known carbapenem resistance mechanisms and undescribed CST and TGC resistance mechanisms were subjected to broth microdilution and meropenem (MEM) disc synergy test in the presence and absence of carbonyl cyanide m-chlorophenylhydrazine (CCCP), a H(+) conductor (protonophore). Results and conclusions: Susceptibility to MEM, imipenem (IMP), CST, and TGC was found in only 2, 0, 17, and 9 isolates respectively. Addition of CCCP reversed resistance to CST, TGC, IMP, and MEM in 44, 3, 0, and 0 isolates respectively; CST had the highest mean minimum inhibitory concentration (MIC) fold change (193.12; p < 0.0001) post CCCP compared to that of MEM (1.70), IMP (1.49) and TGC (1.16). Eight isolates tested positive for the MEM-CCCP disc synergy test. We concluded that CCCP reverse CST resistance in CST-resistant Enterobacteriaceae. Although CCCP is an experimental agent with no therapeutic value clinically, further studies are necessary to decipher the mechanisms underlying the CST-CCCP synergy to inform the development of adjuvants that could be therapeutically effective in CST-resistant infections.


