NeoastilbinCAS# 54081-47-9 |
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Isoastilbin
Catalog No.:BCN5719
CAS No.:54081-48-0
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54081-47-9 | SDF | Download SDF |
| PubChem ID | 442437 | Appearance | Powder |
| Formula | C21H22O11 | M.Wt | 450.40 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
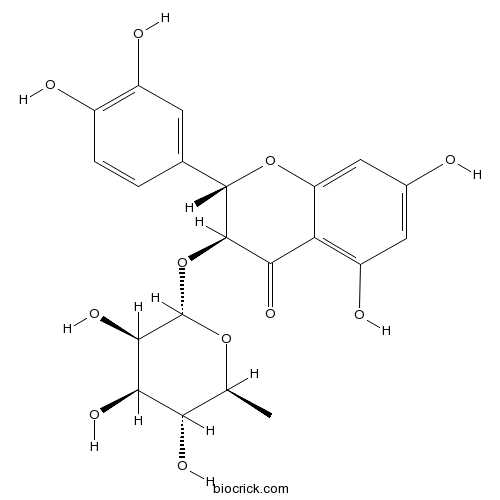
| Chemical Name | (2S,3S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | ||
| Standard InChIKey | ZROGCCBNZBKLEL-MFSALPCASA-N | ||
| Standard InChI | InChI=1S/C21H22O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-26,28-29H,1H3/t7-,15-,17+,18+,19-,20+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Neoastilbin may have antioxidant and anti-inflammatory activities, it shows potent inhibition of lens aldose reductase. |
| Targets | Lens aldose reductase |
| In vitro | Antioxidant and Anti-Inflammatory Activities of Phenolic-Enriched Extracts of Smilax glabra.[Pubmed: 25477999 ]Evid Based Complement Alternat Med. 2014;2014:910438.Smilax glabra Roxb. has been used for a long time as both food and folk medicine. |
| Kinase Assay | Inhibition of aldose reductase by dihydroflavonols in Engelhardtia chrysolepis and effects on other enzymes.[Pubmed: 8698090 ]Experientia. 1996 Jun 15;52(6):564-7.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2004 Sep;29(9):867-70.Studies on dihydroflavonol glycosides from rhizome of Smilax glabra.[Pubmed: 15575206]To investigate the chemical constituents from the rhizomes of Smilax glabra.
|

Neoastilbin Dilution Calculator

Neoastilbin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2202 mL | 11.1012 mL | 22.2025 mL | 44.405 mL | 55.5062 mL |
| 5 mM | 0.444 mL | 2.2202 mL | 4.4405 mL | 8.881 mL | 11.1012 mL |
| 10 mM | 0.222 mL | 1.1101 mL | 2.2202 mL | 4.4405 mL | 5.5506 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.444 mL | 0.8881 mL | 1.1101 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.444 mL | 0.5551 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 27-O-acetyl-withaferin A
Catalog No.:BCN8852
CAS No.:1214886-35-7
- Isorhamnetin 7-O-alpha-L-rhamnoside
Catalog No.:BCN8850
CAS No.:17331-72-5
- Hyperforin
Catalog No.:BCN8848
CAS No.:11079-53-1
- Bacopaside N2
Catalog No.:BCN8847
CAS No.:871706-75-1
- Sterebin E
Catalog No.:BCN8846
CAS No.:114343-74-7
- 4'-Methoxyagarotetrol
Catalog No.:BCN8845
CAS No.:123278-01-3
- Mulberrofuran Q
Catalog No.:BCN8844
CAS No.:101383-35-1
- Hydroxy-beta-sanshool
Catalog No.:BCN8841
CAS No.:97465-69-5
- Iso-sagittatoside A
Catalog No.:BCN8840
CAS No.:503456-08-4
- Celosin J
Catalog No.:BCN8839
CAS No.:1623405-29-7
- Arjunetin
Catalog No.:BCN8838
CAS No.:31297-79-7
- Quercetin 3-O-neohesperidoside
Catalog No.:BCN8837
CAS No.:32453-36-4
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
- Gardoside
Catalog No.:BCN8855
CAS No.:54835-76-6
- Rhaponticin 6''-O-gallate
Catalog No.:BCN8856
CAS No.:94356-23-7
- Hellebrigenin
Catalog No.:BCN8857
CAS No.:465-90-7
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3
- Pangelin
Catalog No.:BCN8861
CAS No.:33783-80-1
- Isoarnebin I
Catalog No.:BCN8862
CAS No.:24502-79-2
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCN8865
CAS No.:53350-26-8
- 7-Ethoxyrosmanol
Catalog No.:BCN8866
CAS No.:111200-01-2
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9


