5,7,3',4',5'-PentamethoxyflavoneCAS# 53350-26-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53350-26-8 | SDF | Download SDF |
| PubChem ID | 493376 | Appearance | Powder |
| Formula | C20H20O7 | M.Wt | 372.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
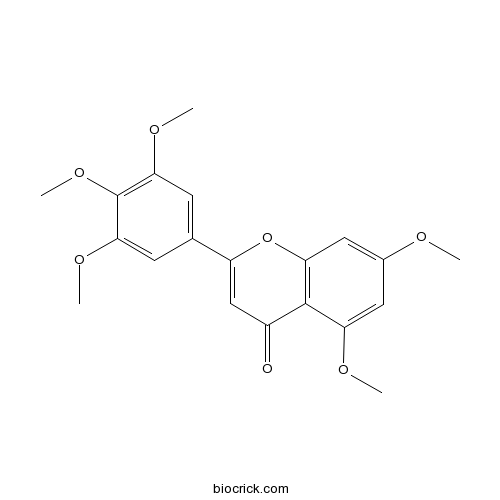
| Chemical Name | 5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC2=C(C(=C1)OC)C(=O)C=C(O2)C3=CC(=C(C(=C3)OC)OC)OC | ||
| Standard InChIKey | GIKVSFNAEBQLGB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20O7/c1-22-12-8-15(23-2)19-13(21)10-14(27-16(19)9-12)11-6-17(24-3)20(26-5)18(7-11)25-4/h6-10H,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3',4',5',5,7-Pentamethoxyflavone has anti-inflammatory and cancer chemopreventive activities. 3',4',5',5,7-Pentamethoxyflavone could be used as an effective adjuvant sensitizer to increase the efficacy of chemotherapeutic drugs by downregulating Nrf2 signaling pathway, it sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway. |
| Targets | P-gp | NO | IL Recepter | Nrf2 | PARP | Caspase | Bcl-2/Bax |
| In vitro | Apoptosis Effects of Dihydrokaempferol Isolated from Bauhinia championii on Synoviocytes.[Pubmed: 30622621 ]Evid Based Complement Alternat Med. 2018 Dec 2;2018:9806160.Bauhinia championii (Benth.) Benth. is a traditional medicinal plant used in China to treat rheumatoid arthritis (RA), especially in She ethnic minority group. This study focused on the active constituents from the rattan of B. championii (Benth.) Benth., which possess potential apoptosis effects.
Flavones as colorectal cancer chemopreventive agents--phenol-o-methylation enhances efficacy.[Pubmed: 19638489 ]Cancer Prev Res (Phila). 2009 Aug;2(8):743-50.Flavonoids occur ubiquitously in plants, and some possess preclinical cancer chemopreventive activity. Little is known about molecular features that mediate chemopreventive efficacy of flavonoids.
|
| Kinase Assay | 3',4',5',5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway.[Pubmed: 25843086 ]Mol Cells. 2015 May;38(5):396-401.Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important redox-sensitive transcription factor that regulates the expression of several cytoprotective genes. More recently, genetic analyses of human tumors have indicated that Nrf2 may cause resistance to chemotherapy.
|
| Cell Research | The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1).[Pubmed: 26710980 ]Pharm Biol. 2016;54(5):868-81.Context Murraya paniculata (L.) Jack (Rutaceae), Qianlixiang in Chinese, is distributed in China. As an important traditional Chinese medicine (TCM), it demonstrates many bioactivities, such as febrifuge, astringent, anti-dysenteric, and tonic.
The objective of this study is to evaluate the anti-inflammatory effect of three flavonoids isolated from M. paniculata in lipopolysaccharide (LPS)-activated murine macrophage cell line and ethanol-induced gastric damage on gastric epithelial cell (GES-1). |
| Structure Identification | Biochem Biophys Res Commun. 2004 Jul 30;320(3):672-9.Reversal of P-glycoprotein-mediated MDR by 5,7,3',4',5'-pentamethoxyflavone and SAR.[Pubmed: 15240100 ]
|

5,7,3',4',5'-Pentamethoxyflavone Dilution Calculator

5,7,3',4',5'-Pentamethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6853 mL | 13.4264 mL | 26.8528 mL | 53.7057 mL | 67.1321 mL |
| 5 mM | 0.5371 mL | 2.6853 mL | 5.3706 mL | 10.7411 mL | 13.4264 mL |
| 10 mM | 0.2685 mL | 1.3426 mL | 2.6853 mL | 5.3706 mL | 6.7132 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5371 mL | 1.0741 mL | 1.3426 mL |
| 100 mM | 0.0269 mL | 0.1343 mL | 0.2685 mL | 0.5371 mL | 0.6713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Isoarnebin I
Catalog No.:BCN8862
CAS No.:24502-79-2
- Pangelin
Catalog No.:BCN8861
CAS No.:33783-80-1
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3
- Hellebrigenin
Catalog No.:BCN8857
CAS No.:465-90-7
- Rhaponticin 6''-O-gallate
Catalog No.:BCN8856
CAS No.:94356-23-7
- Gardoside
Catalog No.:BCN8855
CAS No.:54835-76-6
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
- Neoastilbin
Catalog No.:BCN8853
CAS No.:54081-47-9
- 27-O-acetyl-withaferin A
Catalog No.:BCN8852
CAS No.:1214886-35-7
- Isorhamnetin 7-O-alpha-L-rhamnoside
Catalog No.:BCN8850
CAS No.:17331-72-5
- 7-Ethoxyrosmanol
Catalog No.:BCN8866
CAS No.:111200-01-2
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9
- Cassiaside B
Catalog No.:BCN8868
CAS No.:119170-51-3
- Emodin-1-O-beta-gentiobioside
Catalog No.:BCN8869
CAS No.:849789-95-3
- N-(p-Coumaroyl) serotonin
Catalog No.:BCN8870
CAS No.:68573-24-0
- Quercetin 3-O-sophoroside-7-O-rhamnoside
Catalog No.:BCN8871
CAS No.:64828-40-6
- Hydroxy-alpha-sanshool
Catalog No.:BCN8872
CAS No.:83883-10-7
- Aegeline
Catalog No.:BCN8873
CAS No.:456-12-2
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- N-trans-Sinapoyltyramine
Catalog No.:BCN8875
CAS No.:200125-11-7
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8


